Abstract
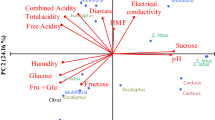
The emergent market for honeydew honeys in Europe prompt to increasing requirements of consumers and honey industry for the characterisation of this type of honey. The aim of this study was to characterise 59 samples of Spanish oak honeydew honeys. Physicochemical properties showed values within the limits established by the legislation and typical for honeydew honeys. Honeys were differentiated into two groups according to the hue (hab) and all were classified as dark honeys (L* < 55). A total of 14 minerals were determined, with K, P, Mg, and Ca being the most abundant. The development and validation of an HPLC method allowed the determination of the contents of two monosaccharides, five disaccharides, and two trisaccharides. Total phenolic and flavonoid contents showed mean values of 130.2 mg/100 g and 11.3 mg/100 g of honey, respectively. Honeydew honeys showed ability to scavenge free radicals and to inhibit lipid peroxidation, which is very interesting, because, as far as we know, there are no previous studies for this type of honey. Results showed that all honeydew honeys are a source of chemical compounds with nutritional and antioxidant properties that could be of interest for consumers and food industry.


Similar content being viewed by others
Abbreviations
- CE:
-
Catechin equivalents
- GAE:
-
Gallic acid equivalents
- HMF:
-
Hydroxymethylfurfural
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- RID:
-
Differential refractive index detector
- TBARS:
-
Thiobarbituric acid reactive substances
- TE:
-
Trolox equivalent
- TEAC:
-
Trolox equivalent antioxidant capacity
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
References
Maschwitz U, Dumpert K, Tuck KR (1986) Ants feeding on anal exudate from tortricid larvae: a new type of trophobiosis. J Nat Hist 20:1041–1050
Delabie JHC (2001) Trophobiosis between formicidae and hemiptera (Sternorrhyncha and Auchenorrhyncha): an overview. Neotrop Entomol 30:501–516
Carter CI, Maslen NR (1982) Conifer lachnids. Forestry commission bulletin, 58th edn. HMSO, London
Binazzi A, Scheurer S (2009) Atlas of the honeydew producing conifer aphids of Europe. Aracne, Rome
Persano Oddo L, Piro R (2004) Main European unifloral honeys: descriptive sheets. Apidologie 35:38–81
Jerković I, Marijanović Z (2010) Oak (Quercus frainetto Ten.) honeydew honey approach to screening of volatile organic composition and antioxidant capacity (DPPH and FRAP Assay). Molecules 15:3744–3756
Rybak-Chmielewska H, Szczęsna T, Waś E, Jaśkiewicz K, Teper D (2013) Characteristics of Polish unifloral honeys IV. honeydew honey, mainly Abies alba L. J Apic Sci 57:51–59
Castro-Vázquez L, Díaz-Maroto MC, Pérez-Coello MS (2006) Volatile composition and contribution to the aroma of Spanish honeydew honeys. Identification of a new chemical marker. J Agric Food Chem 54:4809–4813
Rodríguez Flores MS, Escuredo O, Seijo MC (2015) Assessment of physicochemical and antioxidant characteristics of Quercus pyrenaica honeydew honeys. Food Chem 166:101–106
Krakar D (2012) Characteristics of Croatian oak honeydew honey from the Požega Valley. Radovi Zavoda za znanstveni i umjetniĉki rad u Požegi 1:375–397
Bentabol Manzanares A, Hernández-García Z, Rodríguez-Galdón B, Rodríguez-Rodríguez E, Díaz-Romero C (2011) Differentiation of blossom and honeydew honeys using multivariate analysis on the physicochemical parameters and sugar composition. Food Chem 126:664–672
Simova S, Atanassov A, Shishiniova M, Bankova V (2012) A rapid differentiation between oak honeydew honey and nectar and other honeydew honeys by NMR spectroscopy. Food Chem 134:1706–1710
Pita-Calvo C, Vázquez M (2017) Differences between honeydew and blossom honeys: a review. Trends Food Sci Technol 59:79–87
Vela L, De Lorenzo C, Pérez RA (2007) Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J Sci Food Agric 87:1069–1075
Escuredo O, Míguez M, Fernández-González M, Seijo MC (2013) Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem 138:851–856
Karabagias IK, Vlasiou M, Kontakos S, Drouza C, Kontominas MG, Keramidas AD (2018) Geographical discrimination of pine and fir honeys using multivariate analyses of major and minor honey components identified by 1H NMR and HPLC along with physicochemical data. Eur Food Res Technol 244:1249–1259
Lachman J, Orsák M, Hejtmánková A, Kovárová E (2010) Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT-Food Sci Technol 43:52–58
Escuredo O, Silva LR, Valentão P, Seijo MC, Andrade PB (2012) Assessing Rubus honey value: pollen and phenolic compounds content and antibacterial capacity. Food Chem 130:671–678
Terrab A, Berjano R, Sanchez JA, Gómez Pajuelo A, Díez MJ (2019) Palynological and geographical characterisation of Spanish oak honeydew honeys. Grana 58:63–77
AOAC (2005) AOAC Official method 920175 official methods of analysis, 18th edn. AOAC International, Gaithersburg
Jara-Palacios MJ, Goncalves S, Hernanz D, Heredia FJ, Romano A (2018) Effects of In vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res Int 109:433–439
Terrab A, Escudero ML, González-Miret ML, Heredia FJ (2004) Colour characteristics of honeys as influenced by pollen grain content: a multivariate study. J Sci Food Agric 84:380–386
Jara-Palacios MJ, Hernanz D, González-Manzano S, Santos-Buelga C, Escudero-Gilete ML, Heredia FJ (2014) Detailed phenolic composition of White grape by-products by RRLC/MS and measurement of the antioxidant activity. Talanta 125:51–57
Kus PM, Congiu F, Teper D, Sroka Z, Jerkovi I, Tuberoso CIG (2014) Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT Food Sci Technol 55:124–130
Habib HM, Al Meqbali FT, Kamal H, Souka UD, Ibrahim WH (2014) Bioactive components, antioxidant and DNA damage inhibitory activities of honeys from arid regions. Food Chem 153:28–34
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Ohkawa H, Ohishi N, Yagi K (1979) Assay for the lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Jara-Palacios MJ, Escudero-Gilete ML, Hernández-Hierro JM, Heredia FJ, Hernanz D (2017) Cyclic voltammetry to evaluate the antioxidant potential in winemaking by products. Talanta 165:211–215
StatSoft Inc. (2007) STATISTICA (data analysis software system), v 8.0., Tulsa, OK
Council EU (2002) Council directive 2001/110/EC of 20 December 2001 relating to honey. Off J Eur Commun L10:47–52
Primorac L, Angelkov B, Mandic ML, Kenjeric D, Nedeljko M, Flanjak I, Pirički AP, Arapceska M (2009) Comparison of the Croatian and Macedonian honeydew honey. J Cent Eur Agric 10:263–270
Karabagias IK, Badeka AV, Kontakos S, Karabournioti S, Kontominas MG (2014) Botanical discrimination of Greek unifloral honeys with physico-chemical and chemometric analyses. Food Chem 165:181–190
Can Z, Yildiz O, Sahin H, Turumtay EA, Silici S, Kolayli S (2015) An investigation of Turkish honeys: their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem 180:133–141
Nozal Nalda MJ, Bernal Yagüe JL, Diego Calva JC, Martín Gómez MT (2005) Classifying honeys from the Soria Province of Spain via multivariate analysis. Anal Bioanal Chem 382:311–319
González-Miret L, Terrab A, Hernanz D, Fernández-Recamales MA, Heredia FJ (2005) Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J Agric Food Chem 53:2574–2580
Conti ME, Stripeikis J, Campanella L, Cucina D, Tudino MB (2007) Characterization of Italian honeys (Marche Region) on the basis of their mineral content and some typical quality parameters. Chem Cent J 1:14
Madejczyk M, Baralkiewicz D (2008) Characterization of Polish rape and honeydew honey according to their mineral contents using ICP-MS and F-AAS/AES. Anal Chima Acta 617:11–17
Pisani P, Protano G, Riccobono F (2008) Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem 107:1553–1560
Gamboa-Abril V, Díaz-Moreno C, Figueroa-Ramírez J (2012) Typification of oak (Quercus humboldtti) honeydew honey from Boyaca and Santander. Vitae 19:382–384
Kaygusuz H, Tezcan F, Erim FB, Yildiz O, Sahin H, Can Z, Kolayli S (2016) Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT Food Sci Technol 68:273–279
Karabagias IK, Louppis AP, Kontakos S, Papastephanou C, Kontominas MG (2017) Characterization and geographical discrimination of Greek pine and thyme honeys based on their mineral content, using chemometrics. Eur Food Res Technol 243:101–113
Golob T, Plestenjak A (1999) Quality of slovene honey. Food Technol Biotech 37:195–201
Szczęsna T, Rybak-Chmielewska H, Skubida P (2003) Contribution to the understanding of the phenomenon of “cement” honey. J Apic Sci 47:103–108
Al ML, Daniel D, Moise A, Bobis O, Laslo L, Bogdanov S (2009) Physicochemical and bioactive properties of different floral origin honeys from Romania. Food Chem 112:863–867
Flanjak I, Kenjeric D, Bubalo D, Primorac L (2016) Characterisation of selected Croatian honey types based on the combination of antioxidant capacity, quality parameters, and chemometrics. Eur Food Res Technol 242:467–475
Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM (2009) Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem 114:1438–1443
Alvarez-Suarez JM, Giampieri F, Damiani E, Astolfi P, Fattorini D, Regoli F, Quiles JL, Battino M (2012) Radical-scavenging activity, protective effect against lipid peroxidation and mineral contents of monofloral cuban honeys. Plant Foods Hum Nut 67:31–38
Karabagias IK, Dimitriou E, Kontakos S, Kontominas MG (2016) Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur Food Res Tech 242:1201–1210
Acknowledgements
The authors would like to thank the following Spanish beekeepers for providing the honey samples: AlpuMiel, Apicasfer SL, Apicultura Moisés, Erica Mel SCG, Mel de L’Avi Lluis, Mel Muria, Miel La Puela SL, Mielar SA, Mieles Sala Higón SL, Mielso SL., Montemiel SC, Naturval Apícola SLU, Primo Mendoza SL, Rodríguez Robledo, Sierra Miel SC, and Torrons i Mel Alemany SL. The authors also acknowledge the assistance of the technical staff of Biology Service (SGI, Universidad de Sevilla).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jara-Palacios, M.J., Ávila, F.J., Escudero-Gilete, M.L. et al. Physicochemical properties, colour, chemical composition, and antioxidant activity of Spanish Quercus honeydew honeys. Eur Food Res Technol 245, 2017–2026 (2019). https://doi.org/10.1007/s00217-019-03316-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03316-x