Abstract
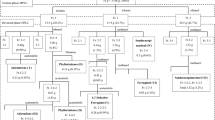
A novel method of stepwise biocatalysis-foam separation-preparative high-performance liquid chromatography (P-HPLC) to extract dioscin of dioscorea zingiberensis. C. H. Wright (DZW) was presented. Compared with the traditional process of direct hydrolysis of DZW, this novel process reduced the non-saponins material entering the hydrolysis, and, as a result, reduced the consumptions of water, acid, and organic solvent significantly, while simultaneously increasing the saponin yield. In addition, the P-HPLC was more efficient, which can not only get high purity, but also the product yield, high speed, simple operation, and it is convenient for industrialized production preparation.




Similar content being viewed by others
References
Liu L, Dong YS, Qi SS, Wang H, Xiu ZL (2009) Biotransformation of steriodal saponins in Dioscorea zingiberensis C.H. Wright to diosgenin by Trichoderma harzianum. Appl Microbiol Biot 85(4):933–940
Qiu LL, Niu H, Huang W (2011) Ultrasonic and fermented pretreatment technology for diosgenin production from Diosorea zingiberensis C. H. Wright. Chem Eng Res Des 89(2):239–247
Huang W, Zhao HZ, Ni JR, Zuo H, Qiu LL, Li H, Li H (2008) The best utilization of D. zingiberensis C. H. Wright by an eco-friendly process. Bioresource Technol 99(15):7407–7411
Corbiere C, Liagre B, Bianchi A, Bordji K, Dauca M, Netter P, Beneytout JL (2003) Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int J Oncol 22(4):899–905
Lei J, Niu H, Li TH, Huang W (2012) A novel β-glucosidase from Aspergillus fumigates releases diosgenin from spirostanosides of Dioscorea zingiberensis C. H. Wright (DZW). World J Microb Biot 28(3):1309–1314
Pires VS, Taketa ATC, Grace G, Schenkel EP (2002) Saponins and sapogenins from brachiaria decumbens stapf. J Brazil Chem Soc 13(2):135–139
Zhang XX, Ito Y, Liang JR, Su Q, Zhang YM, Liu JL, Sun WJ (2013) Preparative isolation and purification of five steroid saponins from Dioscorea zingiberensis C.H.Wright by counter-current chromatography coupled with evaporative light scattering detector. J Pharmaceut Biomed 84(10):117–123
Pang X, Wen D, Zhao Y, Xiong CQ, Wang XQ, Yu LY, Ma BP (2014) Steroidal saponins obtained by biotransformation of total furostanol glycosides from Dioscorea zingiberensis with Absidia coerulea. Carbohyd Res 402:236–240
Zhang XX, Li J, Ito Y, Sun WJ (2014) Simultaneous quantification of five steroid saponins from Dioscorea zingiberensis C.H. Wright in rat plasma by HPLC–MS/MS and its application to the pharmacokinetic studies. Steroids 93:16–24
Wang L, Wang XD, Yuan XF, Zhao B (2011) Simultaneous analysis of diosgenin and sarsasapogenin in Asparagus officinalis byproduct by thin-layer chromatography. Phytochem Analysis 22(1):14–17
Yang H, Chen B, Wang XB, Chue PW, Shen YP, Xia GH, Jia XB (2013) Rapid quantitative analysis of diosgenin in the tubers of Dioscorea zingiberensis C.H. Wright by coupling cellulose enzymolysis and two-phase acid hydrolysis in tandem with HPLC-UV. Nat Prod Res 27(20):1933–1935
Gao ZC, Zhang W, Chen YT, Liu HB, Liu L, Lei L (2016) Optimization of foam separation of Dioscin from Dioscorea zingiberensis C. H. Wright by response surface methodology. Food Sci 37(8):26–31
Dima SO, Meouche W, Dobre T, Nicolescu TV, Sarbu A (2013) Diosgenin-selective molecularly imprinted pearls prepared by wet phase inversion. React Funct Polym 73(9):1188–1197
Xu YW, Han X, Dong DS, Xu LN, Qi Y, Peng JY, Zhan LB (2008) Efficient protocol for purification of diosgenin and two fatty acids from Rhizoma dioscoreae by SFE coupled with high-speed counter-current chromatography and evaporative light scattering detection. J Sep Sci 31(20):3638–3646
Acknowledgements
This research was financially supported by 2017 basic research project Qinghai, China (No. 2017-ZJ-712). The authors thank Prof. Qixiu ZHU of the Key Laboratory of functional organic molecules in Lanzhou University for the assistance in 1HNMR experiments, and Yaling Chi of the Qinghai Normal University for assistance in GC-MS experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that we have no conflict of interest, and the article is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Lei, L., Zhang, W., Gao, Z. et al. Preparation of diosgenin from Dioscorea zingiberensis C. H. Wright by stepwise biocatalysis-foam separation-preparative high-performance liquid chromatography (P-HPLC). Eur Food Res Technol 244, 1447–1452 (2018). https://doi.org/10.1007/s00217-018-3058-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3058-8