Abstract
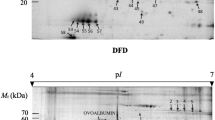
It is a challenging task for the meat industry to search for potential predictors of water-holding capacity (WHC) in meat. Based on the data obtained from cooking loss, longissimus thoracis (LT) of yak can be classified into low cooking loss (LCL) and high cooking loss (HCL) groups. Twenty-six proteins were found to be differentially abundant in the LCL and HCL groups. Results showed that cooking loss can be attributed to structural proteins, metabolic enzymes, stress-related proteins and transport protein. The expression level of desmin, troponin T and l-lactate dehydrogenase increased greatly in the HCL group. We then used western blot and hydrophobicity analysis to validate the representative proteins. Furthermore, prediction of protein subcellular localization revealed that the differentially abundant proteins were mostly positioned in the cytoplasm, nucleus and mitochondria. Accordingly, proteomic and bioinformatic analysis have proven excellent tools to quantify the changes of proteins linked to cooking loss, with which we can explain the processes behind WHC in yak muscle.





Similar content being viewed by others
References
Ding XZ, Liang CN, Guo X, Wu XY, Wang HB, Johnson KA, Yan P (2012) Physiological insight into the high-altitude adaptations in domesticated yaks (Bos grunniens) along the Qinghai-Tibetan Plateau altitudinal gradient. Livest Sci 162:233–239
Zuo HX, Han L, Yu QL, Niu KL, Zhao SN, Shi HM (2016) Proteome changes on water-holding capacity of yak longissimus lumborum during postmortem aging. Meat Sci 121:409–419
Aaslyng MD, Bejerholm C, Ertbjerg P, Bertram HC, Andersen HJ (2003) Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual Prefer 14(4):277–288
Bejerholm C, Aaslyng MD (2004) The influence of cooking technique and core temperature on results of a sensory analysis of pork—Depending on the raw meat quality. Food Qual Prefer 15:19–30
Cheng Q, Sun DW (2008) Factors affecting the water holding capacity of red meat products: a review of recent research advances. Crit Rev Food Sci 48:137–159
Creed PG (1998) Sensory and nutritional aspects for sous vide processed foods. In: Ghazala S (ed) Sous vide and cook chill processing for the food industry. Researchgate, Gaithersburg, pp 57–88
Offer G (1991) Modelling of the formation of pale, soft and exudative meat: Effects of chilling regime and rate and extent of glycolysis. Meat Sci 30:157–184
Wu Z, Bertram HC, Kohler A, Backer U, Ofstad R, Andersen HJ (2006) Influence of aging and salting on protein secondary structures and water distribution in uncooked and cooked pork. A combined FT-IR microspectroscopy and 1H NMR relaxometry study. J Agric Food Chem 54:8589–8597
Franco D, Mato A, Salgado FJ, Lopez-Pedrouso M, Carrera M, Bravo S, Parrado M, Gallardo JM, Zapata C (2015) Tackling proteome changes in the longissimus thoracis bovine muscle in response to pre-slaughter stress. J Proteom 122:73–85
Hwang IH, Park BY, Kim JH, Cho SH, Lee JM (2005) Assessment of postmortem proteolysis by gel-based proteome analysis and its relationship to meat quality traits in pig longissimus. Meat Sci 69:79–91
Suman SP, Rentfrow G, Nair MN, Joseph P (2014) Proteomics of muscle- and species-specificity in meat color stability. J Anim Sci 92:875–882
Wu W, Gao XG, Dai Y, Fu Y, Li XM, Dai RT (2015) Post-mortem changes in sarcoplasmic proteome and its relationship to meat color traits in M. semitendinosus of Chinese Luxi yellow cattle. Food Res Int 72:98–105
Paredi G, Raboni S, Bendixen E, de Almeida AM, Mozzarelli A (2012) “Muscle to meat” molecular events and technological transformations: the proteomics insight. J Proteom 75:4275–4289
Di LA, Elia G, Hamill R, Mullen AM (2013) 2D DIGE proteomic analysis of early post mortem muscle exudate highlights the importance of the stress response for improved water-holding capacity of fresh pork meat. Proteomics 13:1528–1544
Picard B, Lebret B, Cassar-Malek I, Liaubet L, Berri C, Le Bihan-Duval E, Hocquette JF, Renand G (2015) Recent advances in omic technologies for meat quality management. Meat Sci 109:18–26
Zheng AJ, Liu GH, Zhang YS, Hou S, Chang W, Zhang S, Cai H, Chen G (2012) Proteomic analysis of liver development of lean Pekin duck (Anas platyrhynchos domestica). J Proteome Res 75:5396–5413
Wu W, Yu QQ, Fu Y, Tian XJ, Jia F, Li XM, Dai RT (2015) Towards muscle-specific meat color stability of Chinese Luxi yellow cattle: A proteomic insight into post-mortem storage. J Proteom 147:108–118
Serra X, Guerrero L, Guardia MD, Gil M, Sanudo C, Panea B, Campo MM, Olleta JL, Garcia-Cachan MD (2008) Eating quality of young bulls from three Spanish beef breed-production systems and its relationships with chemical and instrumental meat quality. Meat Sci 79:98–104
Warner RD, Ferguson DM, Cottrell JJ, Knee BW (2007) Acute stress induced by the preslaughter use of electric prodders causes tougher beef meat. Aust J Exp Agric 47(7):782–788
Gao XG, Wu W, Ma CW, Li XM, Dai RT (2016) Postmortem changes in sarcoplasmic proteins associated with color stability in lamb muscle analyzed by proteomics. Eur Food Res Technol 242(4):527–535
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Li GS, Li M, Wang JX, Wu JL, Wu FX, Pan Y (2016) Predicting essential proteins based on subcellular localization, orthology and PPI networks. BMC Bioinf 17(8):571–581
Hughes JM, Oiseth SK, Purslow PP, Warner RD (2014) A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci 98(3):520–532
Kondjoyan A, Oillic S, Portanguen SP, Gros JB (2013) Combined heat transfer and kinetic models to predict cooking loss during heat treatment of beef meat. Meat Sci 95:336–344
Polati R, Menini M, Robotti E, Millioni R, Marengo E, Novelli E, Balzan S, Cecconi D (2012) Proteomic changes involved in tenderization of bovine longissimus dorsi muscle during prolonged ageing. Food Chem 135:2052–2069
Puolanne E, Halonen M (2010) Theoretical aspects of water-holding in meat. Meat Sci 86:151–165
Zhang WG, Lonergan SM, Gardner MA, Huff-Lonergan E (2006) Contribution of postmortem changes of integrin, desmin and µ-calpain to variation in water holding capacity of pork. Meat Sci 74:578–585
Huff-Lonergan E, Zhang WG, Lonergan SM (2010) Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci 86:184–195
Marino R, Albenzio M, Malva AD, Caroprese M, Santillo A, Sevi A (2014) Changes in meat quality traits and sarcoplasmic proteins during aging in three different cattle breeds. Meat Sci 98:178–186
Qiu Q, Zhang GJ, Ma T, Qian WB, Wang JY, Ye ZQ, Cao CC, Hu QJ, Kim J, Larkin DM, Auvil L, Capitanu B, Ma J, Lewin HA, Qian XJ, Lang YS, Zhou R, Wang LZ, Wang K, Xia JQ, Liao SG, Pan SK, Lu X, Hou HL, Wang Y, Zang XT, Yin Y, Ma H, Zhang J, Wang ZF, Zhang YM, Zhang DW, Yonezawa T, Hasegawa M, Zhong Y, Liu WB, Zhang Y, Huang ZY, Zhang SX, Long RJ, Yang HM, Wang J, Lenstra JA, Cooper DN, Wu Y, Wang J, Shi P, Wang J, Liu JQ (2012) The yak genome and adaptation to life at high altitude. Nat Genet 44:946–949
Tornberg E (2005) Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci 70(3):493–508
Offer G (1984) Progress in the biochemistry, physiology and structure of meat. In: Proceedings of the 30th European meeting of meat research workers, Bristol, UK, p 87
Koohmaraie M (1992) Effect of pH, temperature, and inhibitors on autolysis and catalytic activity of bovine skeletal muscle mu-calpain. J Anim Sci 70:3071–3080
Hufflonergan E, Lonergan SM (2005) Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci 71(1):194–204
Henckel P, Karlsson A, Jensen MT, Oksbjerg N, Petersen JS (2002) Metabolic conditions in Porcine longissimus muscle immediately pre-slaughter and its influence on peri- and post mortem energy metabolism. Meat Sci 62:145–155
Scheffler TL, Gerrard DE (2007) Mechanisms controlling pork quality development: The biochemistry controlling postmortem energy metabolism. Meat Sci 77:7–16
van de Wiel DFM, Zhang WL (2007) Identification of pork quality parameters by proteomics. Meat Sci 77:46–54
Kristensen L, Purslow PP (2001) The effect of ageing on the water-holding capacity of pork: role of cytoskeletal proteins. Meat Sci 58:17–23
Muroya S, Kitamura S, Tanabe S, Nishimura T, Nakajima I, Chikuni K (2004) N-terminal amino acid sequences of troponin T fragments, including 30 kDa one, produced during postmortem aging of bovine longissimus muscle. Meat Sci 67:19–24
Muroya S, Ohnishi-Kameyama M, Oe M, Nakajima I, Chikuni K (2007) Postmortem changes in bovine troponin-T isoforms on two-dimensional electrophoretic gel analyzed using mass spectrometry and western blotting: The limited fragmentation into basic poly peptides. Meat Sci 75:506–514
Jia XH, Ekman M, Grove H, Faergestad EM, Aass L, Hildrum KI, Hollung K (2007) Proteome changes in bovine longissimus thoracis muscle during the early postmortem storage period. J Proteome Res 6:2720–2731
Carine B, Isabelle CM, Gilles R, Jean-François H (2009) Changes in muscle gene expression related to metabolism according to growth potential in young bulls. Meat Sci 82:205–212
Krischek C, Natter R, Wigger R, Wicke M (2011) Adenine nucleotide concentrations and glycolytic enzyme activities in Longissimus muscle samples of different pig genotypes collected before and after slaughter. Meat Sci 89:217–220
Apaoblaza A, Galaz A, Strobel P, Ramirez-Reveco A, Jerez-Timaure N, Gallo C (2015) Glycolytic potential and activity of adenosine monophosphate kinase (AMPK), glycogen phosphorylase (GP) and glycogen debranching enzyme (GDE) in steer carcasses with normal (< 5.8) or high (> 5.9) 24 h pH determined in M. longissimus dorsi. Meat Sci 101:83–89
Velade A, Gispert M, Faucitano L, Manteca X, Diestre A (2000) The effect of stunning method on the incidence of PSE meat and haemorrhages in pork carcasses. Meat Sci 55:309–314
Di LA, Mullen AM, Elia G, Davey G, Hamill RM (2011) Centrifugal drip is an accessible source for protein indicators of pork ageing and water-holding capacity. Meat Sci 88:261–270
Zapata I, Zerby HN, Wick M (2009) Functional proteomic analysis predicts beef tenderness and the tenderness differential. J Agric Food Chem 57:4956–4963
Foucault G, Vacher M, Cribier S, Arrio-Dupont M (2000) Interactions between beta-enolase and creatine kinase in the cytosol of skeletal muscle cells. Biochem J 346:127–131
Phizicky EM, Fields S (1995) Protein–protein interactions: methods for detection and analysis. Microbiol Rev 59:94–123
Acknowledgements
This work was supported by the program for National Natural Science Foundation of China (No.31460402), National Natural Science Foundation of China (No.31560463) and the National Beef Cattle Industrial Technology System (CARS-38) from the Ministry of Agriculture of the People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Compliance with ethics requirements
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zuo, H., Han, L., Yu, Q. et al. Proteomic and bioinformatic analysis of proteins on cooking loss in yak longissimus thoracis. Eur Food Res Technol 244, 1211–1223 (2018). https://doi.org/10.1007/s00217-018-3037-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3037-0