Abstract
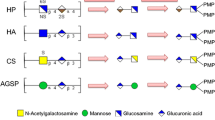
Uronic acid-containing polysaccharides (UACPs) including glycosaminoglycans (GAGs) and some non-GAGs in different tissues of Russian sturgeon were analyzed by detecting characteristic oligosaccharides in their acid hydrolysates after 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatization. Disaccharides without substituents were identified and quantified using HPLC–MS/MS with a triple quadrupole mass spectrometer, while other oligosaccharides including sulfated/acetylated disaccharides and tetrasaccharides were characterized by HPLC–MSn with a linear ion trap mass spectrometer. The results showed that the nostril, gill arch and notochord of Russian sturgeon contained high amounts of CS ranging from 1700 to 3660 mg per 100 g of dried tissues. A non-GAG UACP, named AGSP, with a backbone of →4)-GlcA(1 → 2)-Man(1→, was found in the gill arch, caudal fin and notochord of sturgeon with the contents from 37 to 227 mg per 100 g of dried tissue. Another two unknown non-GAGs, UP1 and UP2, existed in intestine and in body muscles and gill arch, respectively. And an unidentified GAG (UP3) was found in testes, ovaries, swim bladder, intestine and skin. This investigation was the first effort to determine the overall UACP species in sturgeon after extraction of the eggs and caviar production.






Similar content being viewed by others
References
Willför S, Pranovich A, Tarja T, Puls J, Laine C, Suurnäkki A, Saake B, Uotila K, Simolin H, Hemming J, Holmbom B (2009) Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides–A comparison between different hydrolysis and subsequent chromatographic analytical techniques. Ind Crop Prod 29:571–580
Lim JJ, Temenoff JS (2013) The effect of desulfation of chondroitin sulfate on interactions with positively charged growth factors and upregulation of cartilaginous markers in encapsulated MSCs. Biomaterials 34:5007–5018
Neha SG, Ricardo LM (2008) The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des 72:455–482
Flengsrud R, Larsen ML, Ødegaard OR (2010) Purification, characterization and in vivo studies of salmon heparin. Thromb Res 126:e409–e417
Souza MLS, Dellias JMM, Melo FR, Silva LCF (2007) Structural composition and anticoagulant activity of dermatan sulfate from the skin of the electric eel, Electrophorus electricus (L.). Comp Biochem Physiol A: Biochem Mol Biol 147:387–394
Krylov VB, Grachev AA, Ustyuzhanina NE, Ushakova NA, Preobrazhenskaya ME, Kozlova NI (2011) Preliminary structural characterization, anti-inflammatory and anticoagulant activities of chondroitin sulfates from marine fish cartilage. Russ Chem B 60:746–753
Giji S, Arumugam M, Abirami P, Balasubramanian T (2013) Isolation and characterization of hyaluronic acid from the liver of marine stingray Aetobatus narinari. Int J Biol Macromol 54:84–89
Maccari F, Galeotti F, Volpi N (2015) Isolation and structural characterization of chondroitin sulfate from bony fishes. Carbohyd Polym 129:143–147
Gui M, Song JY, Zhang L, Wang S, Wu RY, Ma CW, Li PL (2015) Chemical characteristics and antithrombotic effect of chondroitin sulfates from sturgeon skull and sturgeon backbone. Carbohyd Polym 123:454–460
Murado MA, Montemayor MI, Cabo ML, Vázquez JA, González MP (2012) Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food Bioprod Process 90:491–498
Mansour MB, Majdoub H, Bataille I, Roudesli MS, Hassine M, Ajzenberg N, Chaubet F, Maaroufi RM (2009) Polysaccharides from the skin of the ray Raja radula. Partial characterization and anticoagulant activity. Thromb Res 123:671–678
Zhang Y, Wang ZF, Zhang XR, Zhou WX, Huang LJ (2011) One-pot fluorescent labeling of saccharides with fluorescein-5-thiosemicarbazide for imaging polysaccharides transported in living cells. Carbohyd Res 346:2156–2164
Osago H, Shibata T, Hara N, Kuwata S, Kono M, Uchio Y, Tsuchiya M (2014) Quantitative analysis of glycosaminoglycans, chondroitin/dermatan sulfate, hyaluronic acid, heparan sulfate, and keratan sulfate by LC-ESI-MS/MS. Anal Biochem 467:62–74
Cai XXQ, Lei P, Lamb DH, Shannon A, Jacoby J, Joe Kruk, Kensinger RD, Ryall R, Zablackis E, Cash P (2004) LC/MS characterization of meningococcal depolymerized polysaccharide group C reducing endgroup and internal repeating unit. Anal Chem 76:7387–7390
Li GY, Li LY, Tian F, Zhang LX, Xue CH, Linhardt RJ (2015) Glycosaminoglycanomics of cultured cells using a rapid and sensitive LC-MS/MS approach. ACS Chem Biol 10:1303–1310
Huang Yu, Shi XF, Yu X, Leymarie N, Staples GO, Yin HF, Killeen K, Zaia J (2011) Improved liquid chromatography-MS/MS of heparan sulfate oligosaccharides via chip-Based pulsed makeup flow. Anal Chem 83:8222–8229
Han XQ, Chan CLB, Dong CX, Yang YH, Ko CH, Yue GLG (2011) Isolation, structure characterization, and immunomodulating activity of a hyperbranched polysaccharide from the fruiting bodies of Ganodermasinense. J Agric Food Chem 60:4276–4281
Cao JL, Wen CR, Lu JJ, Teng N, Song S, Zhu BW (2015) Characterization of acidic polysaccharides from the Mollusks through acid hydrolysis. Carbohyd Polym 130:268–274
Wang HX, Zhao J, Li DM, Wen CR, Liu HM, Song S, Zhu BW (2015) Comparison of polysaccharides of Haliotis discus hannai and Volutharpa ampullacea perryi by PMP–HPLC–MSn analysis upon acid hydrolysis. Carbohyd Res 415:48–53
Arima K, Fujita H, Toita R, Imazu A, Tsutsumishita N (2013) Amounts and compositional analysis of glycosaminoglycans in the tissue of fish. Carbohyd Res 366:25–32
Garnjanagoonchorn W, Wongekalak L, Engkagul A (2007) Determination of chondroitin sulfate from different sources of cartilage. Chem Eng Process 46:465–471
Hovingh P, Piepkorn M, Linker A (1986) Biological implications of the structural, antithrombin affinity and anticoagulant activity relationships among vertebrate heparins and heparan sulphates. Biochem J 237:573–581
Maccari F, Ferrarini F, Volpi N (2010) Structural characterization of chondroitin sulfate from sturgeon bone. Carbohyd Res 345:1575–1580
Acknowledgements
The work was supported by China Postdoctoral Science Foundation funded project (No. 2016M591418), Cultivation Plan for Youth Agricultural Science and Technology Innovative Talents of Liaoning Province (No. 2014002), National Key Technology Research and Development Program of China during the 12th Five-Year Plan (No. 2014BAD04B09). We thank Quzhou Sturgeon Science and Technology Development Co., Ltd for support during sample preparation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Guo, L., Wang, P., Liu, B. et al. Identification and quantification of uronic acid-containing polysaccharides in tissues of Russian sturgeon (Acipenser gueldenstaedtii) by HPLC–MS/MS and HPLC–MSn . Eur Food Res Technol 243, 1201–1209 (2017). https://doi.org/10.1007/s00217-016-2834-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2834-6