Abstract
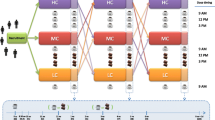
Coffee is the main source of chlorogenic acids (CGAs) in human nutrition. In this study, one main point of interest was whether the simultaneous consumption of coffee and food affects the absorption and bioavailability of CGAs. Fourteen healthy participants consumed pure instant coffee (T1), or coffee with either a high-carbohydrate (T2) or a high-fat meal (T3). All volunteers consumed the same quantity of CGAs (3.1 mg CGA/kg bw), and blood and urine samples were collected at various time points for up to 15 h and 24 h after consumption, respectively. Existing CGAs, and their respective metabolite concentrations, were determined using HPLC–ESI-MS/MS. The area under the curve (AUC) was the measure of CGA quantity in plasma samples collected after each treatment. We observed significantly greater CGA bioavailability after pure instant coffee consumption (T1) than with an additional consumption of a high-fat meal (T3). However, the difference in CGA bioavailability between pure coffee (T1) and coffee plus high-carbohydrate meal (T2) was not statistically significant. The latter observation held true when T3-samples were compared with T2-samples. When the metabolites were split into their respective classes, significant differences in the sums of AUC were observed only for some classes, and not among the treatments. Data did not show differences in the total bioavailability, but revealed differences in the kinetic of release. The co-ingestion of breakfast favored a slow and continuous release of colonic metabolites in contrast to the non-metabolized coffee components appearing in the first hour after coffee consumption. Urine samples collected over 24 h did not show any statistically significant differences among the treatments. Only samples collected within the first 6 h post-coffee consumption showed that the CGA quantities in urine samples collected after T1 and T3 were significantly greater than quantities with an additional consumption of carbohydrates (T2). Breakfast had no significant effect on CGA absorption from coffee. However, a shift in gastrointestinal transit time and in the plasma metabolite composition was observed. So breakfast consumption induces retarded release of chlorogenic acid metabolites in humans.





Similar content being viewed by others
Abbreviations
- CGA:
-
Chlorogenic acid
- 3,4-diCQA:
-
3,4-Di-O-caffeoylquinic acid
- 4,5-diCQA:
-
4,5-Di-O-caffeoylquinic acid
- 5-CQA:
-
5-O-Caffeoylquinic acid
- CA3S:
-
Caffeic-3′-O-sulfate
- CA4S:
-
Caffeic-4′-O-sulfate
- DHCA:
-
Dihydrocaffeic acid
- DHCA3S:
-
Dihydrocaffeic-3′-O-sulfate
- DHFA:
-
Dihydroferulic acid
- DHFA4G:
-
Dihydroferulic-4′-O-glucuronide
- DHFA4S:
-
Dihydroferulic-4′-O-sulfate
- DHiFA:
-
Dihydroisoferulic acid
- DHiFA3G:
-
Dihydroisoferulic-3′-O-glucuronide
- 5-FQA:
-
5-O-Feruloylquinic acid
- 3-FQA:
-
3-O-Feruloylquinic acid
- 4-FQA:
-
4-O-Feruloylquinic acid
- FA4G:
-
Ferulic-4′-O-glucuronide
- FA4S:
-
Ferulic-4′-O-sulfate
- iFA:
-
Isoferulic acid
- iFA3G:
-
Isoferulic-3′-O-glucuronide
- mDHCoA:
-
Dihydro-m-coumaric acid
- mDHCoAS:
-
Dihydro-m-coumaric-3′-O-sulfate
- MeFA:
-
Methylferulic acid
References
Natella F, Nardini M, Giannetti I, Dattilo C, Scaccini C (2002) Coffee drinking influences plasma antioxidant capacity in humans. J Agric Food Chem 50:6211–6216
Zhang W, Lopez-Garcia E, Li TY, Hu FB, van Dam RM (2009) Coffee consumption and risk of cardiovascular diseases and all-cause mortality among men with type 2 diabetes. Diabetes Care 32:1043–1045
Stalmach A, Mullen W, Nagai C, Crozier A (2006) On-line HPLC analysis of the antioxidant activity of phenolic compounds in brewed, paper-filtered coffee. Br J Plant Physiol 18:253–262
Clifford MN (2000) Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric 80:1033–1043
Lemanska K, Szymusiak H, Tyrakowska B, Zielinski R, Soffers AE, Rietjens IM (2001) The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic Biol Med 31:869–881
Nardini M, Cirillo E, Natella F, Scaccini C (2002) Absorption of phenolic acids in humans after coffee consumption. J Agric Food Chem 50:5735–5741
Azuma K, Ippoushi K, Nakayama M, Ito H, Higashio H, Terao J (2000) Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J Agric Food Chem 48:5496–5500
Stalmach A, Mullen W, Barron D, Uchida K, Yokota T, Cavin C, Steiling H, Williamson G, Crozier A (2009) Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Metab Dispos 37:1749–1758
Erk T, Hauser J, Williamson G, Renouf M, Steiling H, Dionisi F, Richling E (2013) Structure- and dose-absorption relationships of coffee polyphenols. BioFactors 40(1):103–112
Plumb GW, Garcia-Conesa MT, Kroon PA, Rhodes M, Ridley S, Williamson G (1999) Metabolism of chlorogenic acid by human plasma, liver, intestine and gut microflora. J Sci Food Agric 79:390–392
Olthof MR, Hollman PC, Buijsman MN, van Amelsvoort JM, Katan MB (2003) Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J Nutr 133:1806–1814
Rechner AR, Bremner P, Bourne LC, Hubbard GP, Rice-Evans CA (2001) Novel biomarkers of the biotransformation of polyphenols in vivo. Free Radical Bio Med 31:36
Ludwig IA, Paz de Peña M, Concepción C, Alan C (2013) Catabolism of coffee chlorogenic acids by human colonic microbiota. BioFactors 39(6):623–632
Monteiro M, Farah A, Perrone D, Trugo LC, Donangelo C (2007) Chlorogenic acid compounds from coffee are differentially absorbed and metabolized in humans. J Nutr 137:2196–2201
Farah A, Monteiro M, Donangelo CM, Lafay S (2008) Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr 138:2309–2315
Erk T, Williamson G, Renouf M, Marmet C, Steiling H, Dionisi F, Barron D, Melcher R, Richling E (2012) Dose-dependent absorption of chlorogenic acids in the small intestine assessed by coffee consumption in ileostomists. Mol Nutr Food Res 56:1488–1500
Renouf M, Marmet C, Guy P, Fraering AL, Longet K, Moulin J, Enslen M, Barron D, Cavin C, Dionisi F, Rezzi S, Kochhar S, Steiling H, Williamson G (2010) Nondairy creamer, but not milk, delays the appearance of coffee phenolic acid equivalents in human plasma. J Nutr 140:259–263
Scherbl D, Muentnich S, Richling E (2014) In vitro absorption studies of chlorogenic acids from coffee using the Ussing chamber model. Food Res Int 63(Part C):456–463
Booth AN, Emerson OH, Jones FT, DeEds F (1957) Urinary metabolites of caffeic and chlorogenic acids. J Biol Chem 229:51–59
Fischer E (1902) Über die Ester der Aminosäuren. De Gruyter, Berlin
Jackson AS, Pollock ML, Ward A (1980) Generalized equations for predicting body density of women. Med Sci Sports Exerc 12:175–181
Marmet C, Actis-Goretta L, Renouf M, Giuffrida F (2014) Quantification of phenolic acids and their methylates, glucuronides, sulfates and lactones metabolites in human plasma by LC–MS/MS after oral ingestion of soluble coffee. J Pharm Biomed Anal 88:617–625
Bakuradze T, Lang R, Hofmann T, Eisenbrand G, Schipp D, Galan J, Richling E (2015) Consumption of a dark roast coffee decreases the level of spontaneous DNA strand breaks: a randomized controlled trial. Eur J Nutr 54:149–156
Montoya GA, Bakuradze T, Eirich M, Erk T, Baum M, Habermeyer M, Eisenbrand G, Richling E (2014) Modulation of 3′,5′-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br J Nutr 112:1427–1437
Erk T, Williamson G, Renouf M, Marmet C, Steiling H, Dionisi F, Barron D, Melcher R, Richling E (2012) Dose-dependent absorption of chlorogenic acids in the small intestine assessed by coffee consumption in ileostomists. Mol Nutr Food Res 56:1488–1500
Guy PA, Renouf M, Barron D, Cavin C, Dionisi F, Kochhar S, Rezzi S, Williamson G, Steiling H (2009) Quantitative analysis of plasma caffeic and ferulic acid equivalents by liquid chromatography tandem mass spectrometry. J Chromatogr B 877:3965–3974
Renouf M, Guy PA, Marmet C, Fraering AL, Longet K, Moulin J, Enslen M, Barron D, Dionisi F, Cavin C, Williamson G, Steiling H (2010) Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: small intestine and colon are key sites for coffee metabolism. Mol Nutr Food Res 54:760–766
Matsui Y, Nakamura S, Kondou N, Takasu Y, Ochiai R, Masukawa Y (2007) Liquid chromatography-electrospray ionization-tandem mass spectrometry for simultaneous analysis of chlorogenic acids and their metabolites in human plasma. J Chromatogr B 858:96–105
Farrell TL, Dew TP, Poquet L, Hanson P, Williamson G (2011) Absorption and metabolism of chlorogenic acids in cultured gastric epithelial monolayers. Drug Metab Dispos 39:2338–2346
Konishi Y, Zhao Z, Shimizu M (2006) Phenolic acids are absorbed from the rat stomach with different absorption rates. J Agric Food Chem 54:7539–7543
Da Encarnação JA, Farrell TL, Ryder A, Kraut NU, Williamson G (2015) In vitro enzymic hydrolysis of chlorogenic acids in coffee. Mol Nutr Food Res 59(231):239
Royal Society of Chemistry—ChemSpider: The free chemical database. http://www.chemspider.com/Chemical-Structure.1405788.html. Accessed June 2015
Konishi Y, Hitomi Y, Yoshida M et al (2005) Absorption and bioavailability of artepillin C in rats after oral administration. J Agric Food Chem 53:9928–9933
Murota K, Terao J (2003) Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochemi Biophys 417:12–17
Jacobsen C, Schwarz K, Stöckmann H, Eyer AS, Adler-Nissen J (1999) Partitioning of selected antioxidants in mayonnaise. J Agr Food Chem 47:3601–3610
Poquet L, Clifford MN, Williamson G (2008) Transport and metabolism of ferulic acid through the colonic epithelium. Drug Metab Dispos 36:190–197
Lekse JM, Xia L, Stark J, Morrow JD, May JM (2001) Plant catechols prevent lipid peroxidation in human plasma and erythrocytes. Mol Cell Biochem 226:89–95
Silva FAM, Borges F, Guimarães C, Lima JLFC, Matos C, Reis S (2000) Phenolic acids and derivatives: studies on the relationship among structure, radical scavenging activity, and physicochemical parameters†. J Agric Food Chem 48:2122–2126
Roche M, Dufour C, Mora N, Dangles O (2005) Antioxidant activity of olive phenols: mechanistic investigation and characterization of oxidation products by mass spectrometry. Org Biomol Chem 3:423–430
Williamson G, Dionisi F, Renouf M (2011) Flavanols from green tea and phenolic acids from coffee: critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol Nutr Food Res 55:864–873
Renouf M, Guy P, Marmet C, Longet K, Fraering AL, Moulin J, Barron D, Dionisi F, Cavin C, Steiling H, Williamson G (2010) Plasma appearance and correlation between coffee and green tea metabolites in human subjects. Br J Nutr 104:1635–1640
Lang R, Dieminger N, Beusch A, Lee Y-M, Dunkel A, Suess B, Skurk T, Wahl A, Hauner H, Hofmann T (2013) Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal Bioanal Chem 405:8487–8503
Zhao Z, Egashira Y, Sanada H (2004) Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J Nutr 134:3083–3088
Acknowledgements
We thank Ingrid Hemm, Annika Witt, Dr. Nico Watzek, Sylvia Schmidt, Dr. Gina Montoya Para, and Lena Kuenzler for their help and support during this study. We would like to thank Nestlé Research Center (NRC), Lausanne, Switzerland, for providing essential reagents and materials as well as founding this clinical trial. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Some authors are affiliated with Nestlé Research Center (NRC).
Compliance with ethics requirements
The research involved human subjects. The study was approved by the Landesärztekammer Rheinland-Pfalz (no. 837.014.11 (7556)).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scherbl, D., Renouf, M., Marmet, C. et al. Breakfast consumption induces retarded release of chlorogenic acid metabolites in humans. Eur Food Res Technol 243, 791–806 (2017). https://doi.org/10.1007/s00217-016-2793-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2793-y