Abstract
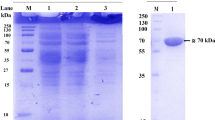
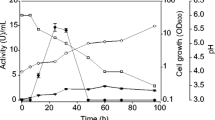
Fructooligosaccharides (FOS) have widely used for the manufacture of low-calorie and functional foods, because they can inhibit intestinal pathogenic microorganism growth and increase Ca2+ and Mg2+ absorption. In this study, the fructosyltransferase (FruSG) from Aspergillus niger strain SG610, which is used in the industrial production of FOS, was successfully overexpressed in Pichia pastoris strain GS115 and characterized. Recombinant FruSG had optimum temperature and pH of 50 °C and 5.5, respectively, and it was stable below 40 °C and at from pH 3.0 to 8.0. K m and k cat values of the recombinant FruSG were 424.5 mmol L−1 and 3.8 × 103 min−1, respectively. On addition of 50 and 100 g L−1 glucose, the K i was 130.6 and 170.0 mmol L−1, respectively. The recombinant FruSG had the highest activity (2294.7 U mL−1) after induction for 144 h in a 5-L fermenter, which was 956.1-fold higher than that of native FruSG from A. niger SG610, and produced a high yield of FOS (64.5 %, w/w). The recombinant FruSG was activated by 5 mmol L−1 Mg2+ and 1 mmol L−1 Fe2+ (relative activity of 125.2 and 120.9 %, respectively), indicating that the enzyme was Mg2+ and Fe2+ dependent. Moreover, toluene, n-butanol, trichloromethane, and ethyl acetate significantly activated FruSG. Together, these results indicate that the recombinant FruSG is an excellent candidate for the industrial production of FOS.





Similar content being viewed by others
References
Alméciga-Díaz CJ, Gutierrez AM, Bahamon I, Rodriguez A, Rodriguez MA, Sanchez OF (2011) Computational analysis of the fructosyltransferase enzymes in plants, fungi and bacteria. Gene 484(1–2):26–34
Guio F, Rodriguez M, Almeciga-Diaz CJ, Sanchez OF (2009) Recent trends in fructooligosaccharides production. Recent Patents Food Nutr Agric 1(3):221–230
Ganaie MA, Lateef A, Gupta US (2014) Enzymatic trends of fructooligosaccharides production by microorganisms. Appl Biochem Biotechnol 172(4):2143–2159
Han Y, Chen L, Mao D, Tang L, Guan L (2010) Expression and activity analysis of sucrose: sucrose 1-fructosyltransferase from onion. New Biotechnol 27(4):324–329
Chuankhayan P, Hsieh CY, Huang YC, Hsieh YY, Guan HH, Hsieh YC, Tien YC, Chen CD, Chiang CM, Chen CJ (2010) Crystal structures of Aspergillus japonicus fructosyltransferase complex with donor/acceptor substrates reveal complete subsites in the active site for catalysis. J Biol Chem 85(30):23249–23262
Morales-Arrieta S, Rodriguez ME, Segovia L, Lopez-Munguia A, Olvera-Carranza C (2006) Identification and functional characterization of levS, a gene encoding for a levansucrase from Leuconostoc mesenteroides NRRL B-512F. Gene 376(1):59–67
Alvarado-Huallanco MB, Maugeri-Filho F (2010) Kinetics and modeling of fructo-oligosaccharide synthesis by immobilized fructosyltransferase from Rhodotorula sp. J Chem Technol Biotechnol 85(12):1654–1662
Salinas MA, Perotti NI (2009) Production of fructosyltransferase by Aureobasidium sp. ATCC 20524 in batch and two-step batch cultures. J Ind Microbiol Biotechnol 36(1):39–43
Onderkova Z, Bryjak J, Vankova K, Polakovic M (2010) Kinetics of thermal inactivation of free Aureobasidium pullulans fructosyltransferase. Enzyme Microb Technol 47(4):134–139
Lateef A, Oloke JK, Gueguim-Kana EB, Raimi OR (2012) Production of fructosyltransferase by a local isolate of Aspergillus niger in both submerged and solid substrate media. Acta Alimentaria 41(1):100–117
L’Hocine L, Wang Z, Jiang B, Xu SY (2000) Purification and partial characterization of fructosyltransferase and invertase from Aspergillus niger AS0023. J Biotechnol 81(1):73–84
van Hijum S, van Geel-Schutten GH, Rahaoui H, van der Maarel M, Dijkhuizen L (2002) Characterization of a novel fructosyltransferase from Lactobacillus reuteri that synthesizes high-molecular-weight inulin and inulin oligosaccharides. Appl Environ Microbiol 68(9):4390–4398
Chen DL, Tong X, Chen SW, Chen S, Wu D, Fang SG, Wu J, Chen J (2010) Heterologous expression and biochemical characterization of alpha-glucosidase from Aspergillus niger by Pichia pastroris. J Agric Food Chem 58(8):4819–4824
Liu L, Yang HQ, Shin Hd, Chen RR, Li JH, Du GC, Chen J (2013) How to achieve high-level expression of microbial enzymes: strategies and perspectives. Bioengineering 4(4):212–223
Jia DX, Li JH, Liu L, Zhang DX, Yang Y, Du GC, Chen J (2013) High-level expression, purification, and enzymatic characterization of truncated poly(vinyl alcohol) dehydrogenase in methylotrophic yeast Pichia pastoris. Appl Microbiol Biotechnol 97(3):1113–1120
Wang ZH, Wang Y, Zhang DX, Li JH, Hua ZZ, Du GC, Chen J (2010) Enhancement of cell viability and alkaline polygalacturonate lyase production by sorbitol co-feeding with methanol in Pichia pastoris fermentation. Bioresour Technol 101(4):1318–1323
Cregg JM, Cereghino JL, Shi JY, Higgins DR (2000) Recombinant protein expression in Pichia pastoris. Mol Biotechnol 16(1):23–52
Wang HL, Li JH, Liu L, Li XM, Jia DX, Du GC, Chen J, Song JN (2012) Increased production of alkaline polygalacturonate lyase in the recombinant Pichia pastoris by controlling cell concentration during continuous culture. Bioresour Technol 124:338–346
Jin H, Liu GQ, Ye XF, Duan ZY, Li Z, Shi ZP (2010) Enhanced porcine interferon-α production by recombinant Pichia pastoris with a combinational control strategy of low induction temperature and high dissolved oxygen concentration. Biochem Eng J 52(1):91–98
Driouch H, Roth A, Dersch P, Wittmann C (2010) Optimized bioprocess for production of fructofuranosidase by recombinant Aspergillus niger. Appl Microbiol Biotechnol 87(6):2011–2024
Park YK, Almeida MM (1991) Production of fructooligosaccharides from sucrose by a transfructosylase from Aspergillus niger. World J Microbiol Biotechnol 7(3):331–334
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Alvarado-Huallanco MB, Maugeri-Filho F (2011) Kinetic studies and modelling of the production of fructooligosaccharides by fructosyltransferase from Rhodotorula sp. Catal Sci Technol 1(6):1043–1050
Duan KJ, Chen JS, Sheu DC (1994) Kinetic-studies and mathematical-model for enzymatic production of fructooligosaccharides from sucrose. Enzyme Microb Technol 16(4):334–339
Rodriguez MA, Sanchez OF, Almeciga-Diaz CJ (2011) Gene cloning and enzyme structure modeling of the Aspergillus oryzae N74 fructosyltransferase. Mol Biol Rep 38(2):1151–1161
Wei T, Yu X, Wang Y, Zhu Y, Du C, Jia C, Mao D (2014) Purification and evaluation of the enzymatic properties of a novel fructosyltransferase from Aspergillus oryzae: a potential biocatalyst for the synthesis of sucrose 6-acetate. Biotechnol Lett 36(5):1015–1020
Fujishima M, Sakai H, Ueno K, Takahashi N, Onodera S, Benkeblia N, Shiomi N (2005) Purification and characterization of a fructosyltransferase from onion bulbs and its key role in the synthesis of fructo-oligosaccharides in vivo. New Phytol 165(2):513–524
Yang HQ, Liu L, Li JH, Du GC, Chen J (2015) Rational design to improve protein thermostability: recent advances and prospects. ChemBioEng Rev 2(2):87–94
Alvaro-Benito M, de Abreu M, Portillo F, Sanz-Aparicio J, Fernandez-Lobato M (2010) New insights into the fructosyltransferase activity of Schwanniomyces occidentalis beta-fructofuranosidase, emerging from nonconventional codon usage and directed mutation. Appl Environ Microbiol 76(22):7491–7499
Alvarado M, Maugeri F (2007) Kinetic modelling of fructooligosaccharide synthesis using a Rhodotorula sp fructosyltransferase. J Biotechnol 131(2):S91–S92
van Hijum S, Bonting K, van der Maarel M, Dijkhuizen L (2001) Purification of a novel fructosyltransferase from Lactobacillus reuteri strain 121 and characterization of the levan produced. FEMS Microbiol Lett 205(2):323–328
Hernalsteens S, Maugeri F (2008) Purification and characterisation of a fructosyltransferase from Rhodotorula sp. Appl Microbiol Biotechnol 79(4):589–596
Ghazi I, Fernandez-Arrojo L, Garcia-Arellano H, Ferrer M, Ballesteros A, Plou FJ (2007) Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J Biotechnol 128(1):204–211
Bravo Rodríguez V, Jurado Alameda E, Martínez Gallegos JF, Reyes Requena A, García López AI et al (2006) Modification of the activity of an alpha-amylase from Bacillus licheniformis by several surfactants. Electron J Biotechnol 9:0717–3458
Russell GL, Britton LN (2002) Use of certain alcohol ethoxylates to maintain protease stability in the presence of anionic surfactants. J Surfactants Deterg 5:5–10
Guo H, Karplus M (1994) Solvent influence on the stability of the peptide hydrogen bond: a supramolecular cooperative effect. J Phys Chem 98:7104–7105
Acknowledgments
This project was financially supported by the Open Project Program of the Key Laboratory of Industrial Biotechnology, Ministry of Education, China (KLIB-KF201301, KLIB-KF201404), the Open Project Program of the Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, China (KLCCB-KF201506), the Fundamental Research Funds for the Central Universities (JUSRP11429), the 111 Project (111-2-06), Natural Science Foundation of Jiangsu Province (BK20140152), National Natural Science Foundation of China (21406089), 863 Program (2014AA021304), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Jiangsu Province “Collaborative Innovation Center for Advanced Industrial Fermentation” industry development program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest declared.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Wenwen Guo and Haiquan Yang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, W., Yang, H., Qiang, S. et al. Overproduction, purification, and property analysis of an extracellular recombinant fructosyltransferase. Eur Food Res Technol 242, 1159–1168 (2016). https://doi.org/10.1007/s00217-015-2620-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2620-x