Abstract
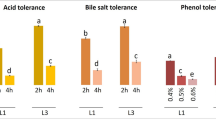
Eighteen dairy starter cultures, spoilage and food-borne pathogenic strains were analyzed for susceptibility to antimicrobial peptides pediocin PA-1 (PedPA-1) and nisin, through the individual 50 % inhibitory concentrations (IC50) determination. The IC50 of purified PedPA-1 was found to be more potent than nisin against five spoilage and food-borne pathogenic strains, i.e., Bacillus cereus NCDC 240, Enterococcus faecalis NCDC 114, Enterococcus faecium NCDC 124, Streptococcus agalactiae NCDC 208 and Staphylococcus aureus NCDC 110. The IC50 of PedPA-1 and nisin ranged from 6.58 to 0.29 µM and 18.91 to 0.03 µM, respectively. Further, PedPA-1 producing Pediococcus pentosaceus NCDC 273 and Pediococcus acidilactici NCDC 252 strains were evaluated for potential probiotic attributes by in vitro studies. Both pediococci strains were able to survive at low pH and 2 % bile with a good bile salt hydrolase activity, cell surface hydrophobicity and β-galactosidase activity that makes them potentially good candidates for probiotics. These strains with proven promising probiotic attributes are good candidates for further investigation through in vivo studies to elucidate their potential health benefits.
Similar content being viewed by others
References
Begley M, Hill C, Gahan CGM (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72:1729–1738
Billot-Klein D, Gutmann L, Sable S, Guittet E, van Heijenoort J (1994) Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus D366 and of the naturally glycopeptide resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol 176:2398–2405
Bustos AY, Saavedra L, de Valdez GF, Raya RR, Taranto MP (2012) Relationship between bile salts hydrolase activity, changes in the internal pH and tolerance to bile acids in lactic acid bacteria. Biotechnol Lett 34:1511–1518
Cabo ML, Murado MA, Gonzalez MP, Pastoriza L (1999) A method for bacteriocin quantification. J Appl Microbiol 87:907–914
Cintas LM, Casaus P, Fernández MF, Hernández PE (1998) Comparative antimicrobial activity of enterocin L50, pediocin PA-1, nisin A and lactocin S against spoilage and foodborne pathogenic bacteria. Food Microbiol 15:289–298
Clark PA, Cotton LN, Martin JH (1997) Selection of bifidobacteria for use as delivery adjuncts in cultured dairy foods. II. Tolerance to stimulated pH of human stomachs. Cult Dairy Prod J 28(4):11–14
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Clinical and Laboratory Standards Institute (CLSI) (2005) Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; proposed guideline. CLSI document M$%-P [ISBN 1-56238-583-6]. CLSI, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA
Conway PL, Gorbach SL, Goldin BR (1987) Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci 70:1–12
Daeschel MA (1990) Application of bacteriocins in food system. In: Bills DD, King SD (eds) Biotechnology and food safety. Butterworth-Heinemann, Boston, pp 19–103
Danielsen M, Simpson PJ, O’Connor EB, Ross RP, Stanton C (2007) Susceptibility of Pediococcus spp. to antimicrobial agents. J Appl Microbiol 102:384–389
Deegan LH, Cotter PD, Hilla C, Ross P (2006) Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy J 16:1058–1071
Eijsink VGH, Skeie M, Middelhoven PH, Brurberg MB, Nes IF (1998) Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl Environ Microbiol 64:3275–3281
European Food Safety Authority (EFSA) (2005) Opinion of the scientific panel on additives and products or substances used in animal feed on the updating of the criteria used in the assessment of bacteria for resistance to antibiotics of human or veterinary importance (question no. EFSA-Q-2004-079), adopted on 25 May 2005. EFSA J 223:1–12
Food and Agricultural Organization/World Health Organization (FAO/WHO) (2002) Guidelines for the evaluation of probiotics in food. Food and Agricultural Organisation, Ontario
Fernandez MF, Boris S, Berbes C (2003) Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol 94:449–455
Fimland G, Johnsen L, Axelsson L, Brurberg MB, Nes IF, Eijsink VGH, Nissen-Meyer A (2000) A C-terminal disulfide bridge in pediocin-like bacteriocins renders bacteriocin activity less temperature dependent and is a major determinant of the antimicrobial spectrum. J Bacteriol 198(9):2643–2648
Garsa AK, Kumariya R, Sood SK, Kumar A, Kapila S (2014) Bacteriocin production and different strategies for their recovery and purification. Probiotics Antimicrob Proteins 6(1):47–58
Gilliland SE, Staley TE, Bush LJ (1984) Importance of bile tolerance of Lactobacillus acidophilus used as dietary adjunct. J Dairy Sci 67:3045–3051
Hoover DG, Dishart KJ, Hermes MA (1989) Antagonistic effect of Pediococcus spp. against Listeria monocytogenes. Food Biotechnol 3:183–196
Hoover DG, Walsh PM, Kolaetis KM, Daly MM (1988) A bacteriocin produced by Pediococcus species associated with a 5.5-megadalton plasmid. J Food Prot 51:29–31
Huaxi Y, Lanwei Z, Yanfeng T, Xue H, Ming D (2010) A novel method for rapid detection of class IIa bacteriocin-producing lactic acid bacteria. Food Control 21:426–430
Hwanhlem N, Watthanasakphuban N, Riebroy S, Benjakul S, H-Kittikun A, Maneerat S (2010) Probiotic lactic acid bacteria from kung-som: isolation, screening, inhibition of pathogenic bacteria. Int J Food Sci Technol 45:594–601
Indian Council of Medical Research-Department of Biotechnology (ICMR-DBT) (2011) Guidelines for evaluation of probiotics in food. Indian Council of Medical Research, New Delhi
Klare I, Konstabel C, Werner G, Huys G, Vankerckhoven V, Kahlmeter G, Hildebrandt B, Muller-Bertling S, Witte W, Goossens H (2007) Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J Antimicrob Chemother 59:900–912
Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, Yadav H (2012) Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res. doi:10.1155/2012/902917
Ljungh A, Wadström T (2006) Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 7:73–90
Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E (2006) Probiotic potential of Lactobacillus strains isolated from dairy products. Int Dairy J 16:189–199
Mattila-Sandholm T, Matto J, Saarela M (1999) Lactic acid bacteria with health claims—interference and interactions with gastrointestinal flora. Int Dairy J 9:25–35
Messi P, Bondi M, Sabia C, Battini R, Manicardi G (2001) Detection and preliminary characterization of a bacteriocin (plantaricin 35d) produced by Lactobacillus plantarum strain. Int J Food Microbiol 64:193–198
Monteagudo-Mera A, Rodriguez-Aparicio L, Rua J, Martinez-Blanco H, Navasa N, Garcia Armesto MR, Ferrero MA (2012) In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. J Funct Foods 4:531–541
Patel AK, Singhania RR, Pandey A, Chincholkar SB (2010) Probiotic bile salt hydrolase: current developments and perspectives. Appl Biochem Biotechnol 162:166–190
Perez P, Minnaard Y, Disalvo E, Antoni G (1998) Surface properties of bifidobacterial strains of human origin. Appl Environ Microbiol 64(1):21–26
Prakash R, Sinha PR, Sinha RN, Singh B (1997) Adherence of lactobacilli to epithelial cells and hexadecane for use of probiotics. Indian J Dairy Sci 10:43–47
Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, Roberfroid M, Rowland I, Cherbut C, Klaenhammer TR (2003) New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol 37:105–118
Rijnaarts HH, Norde W, Bouwer EJ, Lyklema J, Zehnder AJ (1993) Bacterial adhesion under static and dynamic conditions. Appl Environ Microbiol 59:3255–3265
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Smaoui S, Elleuch L, Bejar W, Karray-Rebai I, Ayadi I, Jaouadi B, Mathieu F, Chouayekh H, Bejar S, Mellouli L (2010) Inhibition of fungi and gram-negative bacteria by bacteriocin BacTN635 produced by Lactobacillus plantarum sp. TN635. Appl Biochem Biotechnol 162:1132–1146
Sandeep B, Gaudana Akhilesh S, Dhanani Bagchi T (2010) Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Br J Nutr 103:1620–1628
Schillinger U, Geisen R, Holzapfel WH (1996) Potential of antagonistic microorganisms and bacteriocins for the biological preservation of foods. Trends Food Sci Technol 71:58–64
Schillinger U, Guigas C, Holzapfel WH (2005) In vitro adherence and other properties of lactobacilli used in probiotic yoghurt-like products. Int Dairy J 15:1289–1297
Schillinger U, Lucke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55:1901–1906
Shobharani P, Agrawal R (2011) A potent probiotic strain from cheddar cheese. Indian J Microbiol 51:251–258
Sica MG, Brugnoni LI, Marucci PL, Cubitto MA (2012) Characterization of probiotic properties of lactic acid bacteria isolated from an estuarine environment for application in rainbow trout (Oncorhynchus mykiss, Walbaum) farming. Antonie Van Leewenhoek 101:869–879
Sood SK, Vijay Simha B, Kumariya R, Garsa AK, Mehla J, Meena S, Lather P (2013) Highly specific culture-independent detection of YGNGV motif-containing pediocin-producing strains. Probiotics Antimicrob Proteins 5:37–42
Tari C, Ustok FI, Harsa S (2009) Optimization of the associative growth of novel yoghurt cultures in the production of biomass, β-galactosidase and lactic acid using response surface methodology. Int Dairy J 19:236–243
Todorov SD, Botes M, Guigas C, Schillinger U, Wiid I, Wachsman MB, Holzapfel WH, Dicks LMT (2008) Boza, a natural source of probiotic lactic acid bacteria. J Appl Microbiol 104:465–477
Todorov SD, Dicks LMT (2004) Influence of growth conditions on the production of a bacteriocin by Lactococcus lactis subsp. lactis ST34BR, a strain isolated from barley beer. J Basic Microbiol 44:305–316
Todorov SD, Dicks LMT (2005) Lactobacillus plantarum isolated from molasses produces bacteriocins active against gram-negative bacteria. Enzyme Microb Technol 36:318–326
Todorov SD, Nyati H, Meincken M, Dicks LMT (2007) Partial characterization of bacteriocin AMA-K, produced by Lactobacillus plantarum AMA-K isolated from naturally fermented milk from Zimbabwe. Food Cont 18:656–664
Vidhyasagar V, Jeevaratnam K (2013) Evaluation of Pediococcus pentosaceus strains isolated from Idly batter for probiotic properties in vitro. J Funct Foods 5:235–243
Vijay Simha B, Sood SK, Kumariya R, Garsa AK (2012) Simple and rapid purification of pediocin PA-1 from Pediococcus pentosaceus NCDC 273 suitable for industrial application. Microbiol Res 167:544–549
Acknowledgments
The research work presented in this paper was partially supported by National Initiative on Climate Resilient Agriculture (NICRA-ICAR, Grant No. 2049/3033) project. We are grateful to the Director, National Dairy Research Institute (NDRI), Karnal, for providing the necessary facilities for this work.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garsa, A.K., Kumariya, R., Kumar, A. et al. In vitro evaluation of the probiotic attributes of two pediococci strains producing pediocin PA-1 with selective potency as compared to nisin. Eur Food Res Technol 239, 491–499 (2014). https://doi.org/10.1007/s00217-014-2243-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2243-7