Abstract
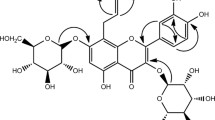
Aceriphyllum rossii Engler (Saxifragaceae) has been used as a nutritious food in Korea, but only minimal studies on this plant have been undertaken. We have examined the estrogenic effect of A. rossii and have discovered its potent constituents. To identify the estrogenic activity of A. rossii, we measured the transactivation of the estrogen-responsive element (ERE) in MCF-7 cells transfected with an ERE-containing construct after treatment of the extract and fractions of A. rossii. A. rossii showed a potent estrogenic activity and gallic acid ethyl ester; quercetin and kaempferol were identified as major constituents of the active fractions by liquid chromatography–nuclear magnetic resonance spectroscopy/mass spectrometry. We confirmed quercetin and kaempferol are the major active constituents after activity-guided isolation of seven compounds: gallic acid ethyl ester (1), quercetin (2), kaempferol (3), quercetin-3-O-(6″-galloyl)-β-d-glucopyranoside (4), quercetin-3-O-β-d-glucopyranoside (5), kaempferol-3-O-(6″-galloyl)-β-d-glucopyranoside (6), and kaempferol-3-O-β-d-glucopyranoside (7). These results show A. rossii could be a possible candidate for the treatment of postmenopausal symptoms.







Similar content being viewed by others
References
Lee CB (1989) Illustrated flora of Korea. Hyangmunsa, Seoul
Han JT, Kim HY, Park YD, Lee YH, Lee KR, Kwon BM, Baek NI (2002) Aceriphyllic acid A, A new ACAT inhibitory triterpenoid, from Aceriphyllum rossii. Planta Med 68:558–561
Lee I, Yoo JK, Na M, Min BS, Lee J, Yun BS, Jin W, Kim H, Youn U, Chen QC, Song KS, Seong YH, Bae K (2007) Cytotoxicity of triterpenes isolated from Aceriphyllum rossii. Chem Pharm Bull 55:1376–1378
Zheng CJ, Sohn MJ, Kim KY, Yu HE, Kim WG (2008) Olean-27-carboxylic acid-type triterpenes with potent antibacterial activity from Aceriphyllum rossii. J Agric Food Chem 56:11752–11756
Min BS, Lee I, Chang MJ, Yoo JK, Na M, Hung TM, Thuong PT, Lee J, Kim JH, Kim JC, Woo MH, Choi JS, Lee HK, Bae K (2008) Anticomplementary activity of triterpenoids from the whole plant of Aceriphyllum rossii against the classical pathway. Planta Med 74:726–729
Zheng CJ, Oh HW, Kim WG (2010) Potent anticariogenic activity of Aceriphyllum rossii and its components, aceriphyllic acid A and 3-oxoolean-12-en-27-oic acid. J Food Sci 75:M78–M82
Han JT, Bang MH, Chun OK, Kim DO, Lee CY, Baek NI (2004) Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch Pharm Res 27:390–395
Cheng J, Zhang C, Shapiro DJ (2007) A functional serine 118 phosphorylation site in estrogen receptor-alpha is required for down-regulation of gene expression by 17beta-estradiol and 4-hydroxytamoxifen. Endocrinology 148:4634–4641
Manavathi B, Kumar R (2006) Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol 207:594–604
Moutsatsou P (2007) The spectrum of phytoestrogens in nature: our knowledge is expanding. Hormones (Athens) 6:173–193
Dontas I, Halabalaki M, Moutsatsou P, Mitakou S, Papoutsi Z, Khaldi L, Galanos A, Lyritis GP (2006) Protective effect of plant extract from Onobrychis ebenoides on ovariectomy-induced bone loss in rats. Maturitas 53:234–242
Wilson ID (2000) Multiple hyphenation of liquid chromatography with nuclear magnetic resonance spectroscopy, mass spectrometry and beyond. J Chromatogr A 892:315–327
Andrade FDP, Santos LC, Datchler M, Albert K, Vilegas W (2002) Use of on-line liquid chromatography-nuclear magnetic resonance spectroscopy for the rapid investigation of flavonoids from Sorocea bonplandii. J Chromatogr A 953:287–291
Kang K, Lee SB, Jung SH, Cha KH, Park WD, Sohn YC, Nho CW (2009) Tectoridin, a poor ligand of estrogen receptor alpha, exerts its estrogenic effects via an ERK-dependent pathway. Mol Cells 27:351–357
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930
Cechinel V, Santos ARS, DeCampos ROP, Miguel OG, Yunes RA, Ferrari F, Messana I, Calixto JB (1996) Chemical and pharmacological studies of Phyllanthus caroliniensis in mice. J Pharm Pharmacol 48:1231–1236
Miguel OG, Cechinel V, Pizzolatti MG, Santos AR, Calixto JB, Ferrari F, Messana I, Yunes RA (1995) A triterpene and phenolic-compounds from leaves and stems of Phyllanthus sellowianus. Planta Med 61:391
Xiao ZP, Wu HK, Wu T, Shi H, Hang B, Aisa HA (2006) Kaempferol and quercetin flavonoids from Rosa rugosa. Chem Nat Comp 42:736–737
Kim HJ, Woo ER, Shin CG, Park H (1998) A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. J Nat Prod 61:145–148
Zhang ZJ, Liao LP, Moore J, Wu T, Wang ZT (2009) Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem 113:160–165
Wong RWK, Rabie ABM (2008) Effect of quercetin on bone formation. J Orthop Res 26:1061–1066
Yang Y, Cao Y, Zhang T-t, Zhu Y, Cao L (2011) The pharmacologic actions progression of quercetin on estrogen related diseases. Guoji Shengzhi Jiankang/Jihua Shengyu Zazhi 30:69–72
Park JS, Rho HS, Kim DH, Chang IS (2006) Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. J Agr Food Chem 54:2951–2956
Masuda T, Iritani K, Yonemori S, Oyama Y, Takeda Y (2001) Isolation and antioxidant activity of galloyl flavonol glycosides from the seashore plant, Pemphis acidula. Biosci Biotech Bioch 65:1302–1309
Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M (2000) Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr 130:1695–1699
Walle T (2004) Absorption and metabolism of flavonoids. Free Radical Bio Med 36:829–837
Day AJ, Canada FJ, Diaz JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MRA, Williamson G (2000) Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett 468:166–170
Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJC, Morgan MRA, Williamson G (1998) Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett 436:71–75
Ioku K, Pongpiriyadacha Y, Konishi Y, Takei Y, Nakatani N, Terao J (1998) Beta-glucosidase activity in the rat small intestine toward quercetin monoglucosides. Biosci Biotech Bioch 62:1428–1431
Acknowledgments
This work was supported by Korea Institute of Science and Technology intramural grant (2Z03850).
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, S.W., Kang, K., Kim, M.A. et al. Phytoestrogenic activity of Aceriphyllum rossii and rapid identification of phytoestrogens by LC–NMR/MS and bioassay-guided isolation. Eur Food Res Technol 239, 237–246 (2014). https://doi.org/10.1007/s00217-014-2212-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2212-1