Abstract
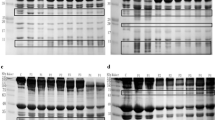
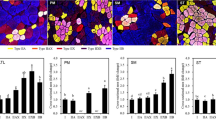
We analyzed differences in some physicochemical parameters of proteins in shoulder rose (M. Cutaneous-omo brachialis) muscle as a result of beef processing to produce pastirma. Samples from processed muscles showed significantly increased concentrations of extracted proteins, especially of H2O P-ex and Guba-Straub–adenosine triphosphate solution (P < 0.01), as a result of the salting–curing process. The salt-curing process was likely to have an important effect on the extractability of muscle proteins such as myosin heavy chain (MHC) and water-soluble proteins, possibly as a result of releasing some proteins from each other and cleaving the structures between certain proteins. The fluorescence intensities of processed samples were higher than those of the control samples at all guanidine hydrochloride concentrations. The hydrophobicity also increased on account of new compounds that were created during the pastirma-making process. Since the process of making pastirma lasts about 4 weeks (drying), the metmyoglobin content was greatly increased in pastirma samples compared with the unprocessed samples (by as much as 57 %). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis results conceived that during processing of pastirma products, MHC and other muscle proteins degraded into polypeptides with smaller molecular weight lower than 15-kDa. The results of this study demonstrated that meat processing promoted the enzymatic digestion of some proteins, and the differences in composition between the control and pastirma samples were thus likely to be owing to protein degradation. The traditional pastirma-making process hence has no negative impact on the structure of the muscle and produces a firmer-textured.







Similar content being viewed by others
References
Gok V, Obuz E, Akkaya L (2008) Effects of packaging method and storage time on the chemical, microbiological, and sensory properties of Turkish pastirma-A dry cured beef product. Meat Sci 80:335–344
Aksu IM, Kaya M, Ockerman H (2005) Effects of modified atmosphere packaging and temperature on the shelf life of sliced pastirma produced from frozen/thawed meat. J Muscle Foods 16:192–206
Aksu MI, Kaya M (2001) Some microbiological, chemical, and physical characteristics of pastirma marketed in Erzurum. Turk J Vet Anim Sci 25:319–326
Kaban G (2009) Changes in the composition of volatile compounds and in microbiological and physicochemical parameters during pastirma processing. Meat Sci 82:17–23
Flores M, Spanier AM, Toldra F (1998) Flavour analysis of dry-cured ham. In: de Shahidi F (ed) Flavor of meat, meat products and seafood’s. Blackie Academic and Professional, London, pp 320–341
Aktas N, Gurses A (2005) Original research article moisture adsorption properties and adsorption isosteric heat of dehydrated slices of pastirma (Turkish dry meat product). Meat Sci 71:571–576
Ahhmed MA, Kaneko G, Ushio H, Inomata T, Yetim H, Karaman S, Muguruma M, Sakata R (2013) Changes in physicochemical properties of proteins in Kayserian pastirma made from the M. semimembranosus muscle of cows during traditional processing. Food Sci Hum Wellness 2:46–55
Isikli ND, Karababa EA (2005) Rheological characterization of fenugreek paste (çemen). J Food Eng 69:185–190
Ahhmed MA, Kawahara S, Ohta K, Nakade K, Soeda T, Muguruma M (2007) Differentiation in improvements of gel strength in chicken and beef sausages induced by transglutaminase. Meat Sci 76:455–462
Gornall GA, Baradawill JC, David MM (1949) Determination of serum protein by means of the biuret reaction. J Biol Chem 177:751–766
Ahhmed MA, Nasu T, Huy QD, Tomisaka Y, Kawahara S, Muguruma M (2009) Effect of microbial transglutaminase on the natural actomyosin cross-linking in chicken and beef. Meat Sci 82:170–178
Hayakawa I, Kajihara J, Morikawa K, Oda M, Fujio Y (1985) Denaturation of bovine serum albumin (BSA) and ovalbumin by high pressure, heat and chemicals. J Food Sci 50:486–491
Tomita S (1963) Studies on the fluorometric analysis of polyelectrolytes (I) protein. Jpn Natl Chem Lab Ind 58:99–107
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Ahhmed MA, Kuroda R, Kawahara S, Ohta K, Nakade K, Aoki T, Muguruma M (2009) Dependence of microbial transglutaminase on meat type in myofibrillar protein cross-linking. Food Chem 112:354–361
Kawahara S, Ahhmed MA, Ohta K, Nakade K, Muguruma M (2007) Inconsistency in the improvements of gel strength in chicken and pork sausages induced by microbial transglutaminase. Asian-Australian J Anim Sci 20:1285–1291
Krzywicki K (1979) Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci 3(1):1–10
Trout GR (1989) Variation in myoglobin denaturation and color of cooked beef, pork and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate and cooking temperature. J Food Sci 54:536
Trout GR (1990) The rate of metmyoglobin formation in beef, pork, and turkey meat as influenced by pH, sodium chloride, and sodium tripolyphosphate. Meat Sci 28:203–210
Astruc T, Labas R, Vendeuvre LJ, Martin LJ, Taylor GR (2008) Beef sausages structure affected by sodium chloride and potassium lactate. Meat Sci 80:1092–1099
Ahhmed MA, Nasu T, Muguruma M (2009) Impact of transglutaminase on the textural, physicochemical, and structural properties of chicken skeletal, smooth, and cardiac muscles. Meat Sci 83:759–767
Pighin GD, Sancho MA, Gonzalez BC (2008) Effect of salt addition on the thermal behavior of proteins of bovine meat from Argentina. Meat Sci 79:549–556
Yetim H, Sagdic O, Dogan M, Ockerman WH (2006) Sensitivity of three pathogenic bacteria to Turkish çemen paste and its ingredients. Meat Sci 74:354–358
Toldra F, Etherington DJ (1988) Examination of cathepsins B, D, H and L activities in dry-cures hams. Meat Sci 59:531–538
Zhen ZY, He ZF, Li HJ, Zhou GH, Zhang JH (2004) Prospect of study on the Jinhua ham maturating and microorganism fermenting. Sichuan Food Ferment: Chin 40:1–3
Zhao GM, Zhou GH, Wang YL, Xu XL, Huan YJ, Wu JQ (2005) Time-related changes in cathepsin B and L activities during processing of Jinhua ham as a function of pH, salt and temperature. Meat Sci 70:381–388
Zhou G, Zhao G (2007) Biochemical changes during processing of traditional Jinhua ham. Meat Sci 77:114–120
Astiasaran I, Villanueva R, Bell J (1990) Analysis of proteolysis and protein insolubility during the manufacturing of varieties of dry sausage. Meat Sci 28:111–117
Molly K, Demeyer D, Civera T, Verplaetse, A (1996) Lipolysis in a Belgian sausage: relative importance of endogenous and bacterial enzymes. Meat Sci 43:235–244
Toldra F (1998) Proteolysis and lipolysis in flavour development of drycured meat products. Meat Sci 49:101–110
Toldra F, Verplaetse A (1995) Endogenous enzyme activity and quality for raw product processing. In: Liindstrom K, Hansson I, Winklund E (eds) Composition of meat in relation to processing, nutritional and sensory quality. ECCEAMST, Uppsala, Sweden, pp 41–55
Prevolnik M, Skrlep M, Janes J, Velikonja-Bolta S, Skorjanc D, Candek-Potokar M (2011) Accuracy of near infrared spectroscopy for prediction of chemical composition, salt content and free amino acids in dry-cured ham. Meat Sci 88:299–304
Ruiz J, Garcia C, Carmen Diaz M, Cava R, Florencio Tejeda J, Ventanas J (1999) Dry cured Iberian ham non-volatile components as affected by the length of the curing process. Food Res Int 32:643–651
Virgili R, Saccani G, Gabba L, Tanzi E, Bordini CS (2007) Changes of free amino acids and biogenic amines during extended ageing of Italian dry-cured ham. LWT Food Sci Technol 40:871–878
Muguruma M, Tsuruoka K, Katayama K, Erwanto Y, Kawahara S, Yamauchi K et al (2003) Soybean and milk protein modified by transglutaminase improves chicken sausage texture even at reduced levels of phosphate. Meat Sci 63:191–197
Carpenter CE, Cornforth DP, Whittier D (2001) Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci 57:359–363
Faustman C, Phillips A (2001) Measurement of discoloration in fresh meat. Curr Protoc Food Anal Chem F3.3.1–F3.3.13
Tekinsen OC, Dogruer Y, Nizamlıoglu M, Gurbuz U (1999) The possibility of using potassium sorbate in cemen and its effect on the microbial quality of pastrami. Turk J Vet Anim Sci 23:227–235
Swatland HJ (1994) The conversion of muscles to meat. Structure and development of meat animals and poultry. Technomic Publishing Company, Pennsylvania
Saito K, Ahhmed A, Takeda H, Kawahara S, Irie M, Muguruma M (2007) Effects of a humidity-stabilizing sheet on the color and K value of beef stored at cold temperatures. Meat Sci 75:275–282
Saito K, Ahhmed MA, Kawahara S, Sugimoto Y, Aoki T, Muguruma M (2009) Evaluation of the performance of osmotic dehydration sheets on freshness parameters in cold-stored beef biceps femoris muscle. Meat Sci 82:260–265
Luccia DA, Picariello G, Cacae G, Scaloni A, Faccia M, Liuzzi V et al. (2005) Proteomic analysis of water soluble and myofibrillar protein changes occurring in dry-cured hams. Meat Sci 69:479–491
Samaranayaka AGP, Li-Chan CY (2011) Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J Funct Food 3:229–254
Huff-Lonergan E, Lonergan M (2005) Mechanisms of water holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci 71:194–204
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahhmed, A.M., Kaneko, G., Ushio, H. et al. Proteins degradation value in cured meat product made from M. Cutaneous-omo brachialis muscle of bovine. Eur Food Res Technol 238, 387–396 (2014). https://doi.org/10.1007/s00217-013-2109-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-2109-4