Abstract
This study was designed to characterize the stability of hydroxycinnamic acids and caffeine obtained from green coffee beans in the form of lyophilized extract (GCE) during heating after supplementation to model systems with saccharose, potato starch, egg white protein, and sunflower oil. Also the addition of iron ions was used. Systems were prepared as a mixture of GCE with a single substance or in a more complex matrix. Heating was carried out at 180 °C for 0.5 and 1 h. Because of the saccharose content, some systems were heated only at 110 °C. The losses of hydroxycinnamic acids in heated systems ranged from 18 to 84 %, and the caffeine from 1.5 to 10 %. The presence of sunflower oil in the systems had the greatest influence on hydroxycinnamic acids degradation. However, in case of the system of each of the examined substances with the coffee preparation, an increased degradation of hydroxycinnamic acids resulting from the introduction of ferrous ions into the systems was observed. Earlier results concerning antioxidant activity of systems containing GCE allow to conclude that the degradation of hydroxycinnamic acids was weakly related to the decrease in antioxidant activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brewed coffee is consumed for many centuries, due to its stimulating properties, as well as due to health beneficial activities [1]. It exhibits anti-inflammatory and anti-mutagenic effects, which prevent chronic disorders, such as cardiovascular and rheumatologic diseases, and also prevent some types of tumors [2]. Consumption of coffee is helpful in the fight against obesity and limits the effects of type II diabetes [3]. Coffee is a rich source of polyphenols, especially hydroxycinnamic acids in human diet [4]. Polyphenols counteract oxidative changes in food as well as show an effect of reducing the oxidative stress in human body. Coffee extracts have extremely strong antioxidant properties against lipid oxidation, higher than many other food products or common antioxidants [5]. This applies to both roasted and green coffee; however, the latter contains even ten times higher concentration of polyphenols [6].
Plant extracts with high antioxidant properties, including green coffee extract, are used as natural antioxidants, enhancing the oxidative stability of food products, due to the growing lack of consumers’ trust toward synthetic antioxidants. For several years, the antioxidant effect of coffee, mainly roasted, was the subject of numerous studies on extending the shelf life of food products, in particular those containing significant amounts of fat, susceptible to oxidative damage [7–10]. Additionally, the beneficial effect of inhibiting the activity of food enzymes, chelation of transition metals, and an increase in the microbiological safety of products enriched with coffee extract can be achieved [11, 12]. In such applications, relatively low concentrations of coffee extract or isolated coffee antioxidants were analyzed, such as 0.1–0.2 % [13, 14].
Current food production trends related to natural antioxidants include not only the protection of food components from oxidation but also giving products improved pro-health properties [15]. Chlorogenic acid, which is the most important polyphenol component of coffee bean, is highly bioavailable to the human body [16]. This means that coffee extract can be successfully used for such purposes. Caffeine contained in coffee (also in tea, yerba maté, cola nuts, and guarana), but not in fruits, vegetables, cereals, other herbs, spices or legumes, due to its health beneficial properties, gives additional pro-health characteristics to coffee extracts comparing to extracts from mentioned foods [17].
However, to meet this aim, the concentration of antioxidants in products has to be significantly higher than this used for the protection of food lipids from oxidation. The suggested level should amount even to 1 g of polyphenols, especially flavonoids and phenolic acids, in a typical daily serving dose of a product, like 100 g of bread, cookies, or pasta. These assumptions come from a report by Williamson and Holst [18]. They write that the dietary recommendations and regulation for any food enriched in polyphenols should limit their consumption to dietary reference level. The references evaluate that polyphenols are consumed in an amount of 1 g per day in a high polyphenol-rich diet, including drinking few cups of tea and coffee. This is why this level of consumption, based on the epidemiologic studies, is assumed to be favorable and absolutely safe. In a typical diet, the consumption of polyphenols is too low, thus comes the need and attention paid to supplementation with them. When setting the level of supplementation, one may neglect other polyphenol sources in the diet [19]. However, both the changes of hydroxycinnamic acids and their interaction with food components lead to the losses of these antioxidants during food processing. Hence, their final content in product may differ significantly from the amounts entered in the original recipe.
The aim of the work was the initial verification of the possibility of supplementation of heat-treated food products with green coffee extract in quantities having potentially significant nutritional importance, based on the determination of stability of hydroxycinnamic acids introduced with the extract to the heated model systems. The preparation of model systems with varying composition was also made in order to determine the effect of the analyzed food ingredients on coffee hydroxycinnamic acids stability.
Materials and methods
Chemicals and reagents
5-O-caffeoylquinic acid (5-O-CQA), 5-O-feruloylquinic acid (5-O-FQA), benzoic acid (BA), caffeine, and formic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA), acetonitrile from Scharlab (Barcelona, Spain), hexane (99 %) and ferrous chloride from Chempur (Piekary Śląskie, Poland). Ultrapure water (18.2 MΩ cm−1) was obtained from a Millipore Milli-Q Plus purification system (Bedford, MA, USA). All other solvents and chemicals used for the analysis were of analytical grade. Chromacol nylon syringe filters (0.2 μm pore size) were purchased from Chromacol Ltd. (Herts, UK).
Preparation of green coffee extract (GCE)
Green Robusta coffee (Coffea canephora) beans originating from Brazil, dehulled by dry method, purchased from Bero Polska (Gdynia, Poland), were used. Coffee beans were ground in a blade laboratory grinder type WZ-1 (ZBPP, Bydgoszcz, Poland) and sieved to a particle size ranging from 480 to 680 μm. Then, aqueous extract was obtained using the ratio 1:5.75 of ground material to the solvent. Extraction consisted of boiling in a PS-5682 First pressure pot (Vienna, Austria) at 110 °C (0.15 MPa) for 10 min, rapid cooling to ambient temperature in an ice bath, and filtering under vacuum. The extraction conditions were consistent with the earlier authors’ study on obtaining green coffee extract of high antioxidant activity [6]. The extract was then freeze-dried in a DELTA 1-24LSC Christ freeze drier (Osterode am Harz, Germany). For further analysis, the fixe extract was stored at a temperature of −25 °C.
Preparation of model systems
Summary of the data for the preparation of particular systems is enclosed in Table 1. Experiments were conducted using single substances, as well as using complex system of them. GCE was added to model systems at a level of 3 g 100 g−1 in all systems in order to easily compare the results of interactions with examined substances. The concentration was chosen based on the statement that 3 g of GCE, containing about 1 g of polyphenols (freeze-dried GCE analysis, Table 2), represents recommended safe and nutritionally beneficial daily intake [18]. In model systems, egg white protein, saccharose, potato starch, and sunflower oil all obtained from a local market were used. The components of model systems were chosen based on the bakery product recipes, including confectionery. Wheat flour was not used due to its too complex composition for model experiments. In complex systems, the proportions of components were set at equilibrium in order to examine the influence of each of the components on hydroxycinnamic acids stability. Ferrous ions as FeCl2 were used in the experiment at a mean concentration established for cereal and confectionary foods [20]. Ferrous ions were used due to the possible supplementation of such products with iron, which may influence the level of degradation of coffee bean polyphenols. All components of such products are introduced before baking; therefore, GCE was also added to model systems before heating. The experiment was designed as dry heating of homogenous powdered mixture, eventually paste or fluid, when oil was added. Samples were heated at 180 °C for 0.5 and 1 h in a horizontal retort equipped with a stirrer. Heating of systems containing saccharose was performed at temperature of 110 °C because of its caramelization occurring at higher temperatures. The conditions were chosen based on the typical time and temperature of baking of bakery and confectionery goods. In real food products, the caramelization of saccharose in products baked at 180 °C does not take place so intensively as in our experiments due to the fact that in our research, the powdered systems were mixed and the temperature was maintained at a given level in the whole volume. In real food processing, these components are not mixed during heating. However, the mixing was adopted in our study to minimize the temperature gradient.
Hydroxycinnamic acids and caffeine analysis
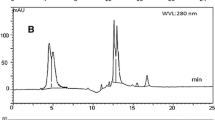
A 5.0 g sample of a model system was defatted three times with 30 mL of hexane for 5 min with constant shaking and subsequently centrifuged for 10 min at 6,000 rpm. Then, the hexane solution was discarded and the residual organic solvent was eliminated under a gentle stream of nitrogen. In case of trials in which sunflower oil accounted for about 50 % of the sample or more, the defatting stage was omitted. The defatted sample was mixed with 100 μL of internal standard solution (benzoic acid, 10 mg mL−1). Hydroxycinnamic acids and caffeine (Fig. 1) were extracted three times from defatted systems with 100 mL of Milli-Q Plus water, for 30 min at ambient temperature in an orbital shaker. After each extraction, the mixture was centrifuged as above and the supernatant was decanted. Supernatants from centrifuged tubes were combined and filtered through a 0.20-μm nylon syringe filters. In order to determine the hydroxycinnamic acids profile in GCE, the freeze-dried preparation was diluted 20-fold with Milli-Q Plus water and filtered as above.
Chromatographic analysis UHPLC/DAD was carried out by using a UHPLC+ Ultimate 3000 system with an autosampler and a diode array detector (DAD) from Dionex (CA, USA). The analytical column Accucore™ C18 (100 mm × 3.0 mm × 2.6 μm) from Thermo Scientific (PA, USA) was used. The mobile phase consisted of 1 % formic acid (solvent A) and acetonitrile/1 % formic acid (80:20, v/v) (solvent B) with flow rate of 0.5 mL min−1. The elution was performed with a gradient starting at 5 % B to reach 35 % B at 23 min and becoming isocratic for 5 min. 2 μL injection volume and 25 °C column temperature were used. Detection was performed with two wavelengths, 280 nm for caffeine and 325 for hydroxycinnamic acids (Fig. 2). Phenolic acids and caffeine were identified by comparing their retention times and UV spectra including λ max absorptions with those of reference standards (caffeine and 5-O-CQA) or with literature data [6, 21]. The quantification was performed using calibration curves of reference substances combined with the internal standard method. Standard calibration curves were obtained for the concentration range of 1–10 mg L−1 of 5-O-CQA, caffeine, or benzoic acid (internal standard). All caffeic acid esters were quantified as 5-O-CQA equivalents.
Statistical analysis
Each model system was prepared twice. Analyses were carried out in triplicate and their results were subjected to statistical analysis. It comprised determination of average values of six measurements and their standard deviation as well as one-way ANOVA (analysis of variation) at the significance level P < 0.05. Also the Pearson correlation coefficients for the relation between the degradation of hydroxycinnamic acids or caffeine and the antioxidative activity presented in earlier reports were calculated.
Results and discussion
For the first time, the degradation of hydroxycinnamic acids extracted from green coffee beans added to model systems containing selected food components alone and in various combinations and subjected to heating was examined. In all systems, significant degradation of hydroxycinnamic acids was observed (P < 0.05) (Table 2), which may result both from the polyphenol changes under the influence of heating with access of oxygen, as well as their interaction with the tested food ingredients. Polyphenols form, for example, adducts with proteins as a result of both non-covalent and covalent interactions. Covalent interactions of proteins and polyphenols oxidized to quinones decrease the number of free primary amino groups of the proteins, cause protein dimerization, and reduce their solubility. Side chains of lysine and tyrosine in proteins appeared the most susceptible residues to react with oxidized polyphenols [22–24]. Non-covalent interactions including hydrogen and ionic bonds and hydrophobic interactions cause an increase in proteins denaturation enthalpy and temperature and this type of bonding may be digested by human digestive tract enzymes and the released hydroxycinnamic acids may be active as antioxidants in the body [25]. Polyphenols heated with carbohydrates may deactivate the radicals generated through decomposition of these substances, thereby limiting the contribution of carbohydrate derivatives in non-enzymatic browning products [26]. The anti-radical activity is shown by polyphenols also in food products containing easily oxidizable lipids, where they scavenge lipid hydrogen peroxide radicals, terminating the chain reaction leading to autooxidation of unsaturated fatty acids. As a result of free radical scavenging, polyphenols transform into low activity phenoxyl radicals. The reactions of polyphenols with carbohydrate and lipid radicals as well as with proteins through covalent interactions lead to irreversible losses of hydroxycinnamic acids.
Single substance model systems
The losses of hydroxycinnamic acids in the analyzed model systems varied from about 18 to 84 %. In case of systems containing single substances, some week trends of polyphenols degradation can be noticed. Degradation of hydroxycinnamic acid increased as a result of heating for a longer time, but larger losses were observed during the first 30 min of heating as compared to the same second period. The smallest losses were found during heating of saccharose with GCE, in the range from 19 to 41 %, probably resulting from applied lower temperature. A similar level of degradation was also found in GCE systems heated with egg white protein, i.e., 18–43 %, despite the fact that in this type of systems, relatively high losses of hydroxycinnamic acids resulting from the polyphenol-protein interactions were expected. Higher levels of hydroxycinnamic acids degradation than in the case of the first two substances have occurred during heating of GCE with potato starch, reaching a level of 25–56 %. However, the greatest green coffee polyphenols degradation took place during the heating of sunflower oil with GCE. In this case, it amounted from 76 to 84 %.
Model systems containing iron ions
There was a negative influence of the presence of ferrous ions on the stability of green coffee polyphenols, probably as an effect of chelating [11]. In case of adding iron ions to single substances, an increase in hydroxycinnamic acids degradation was observed, the most significant in the system with starch. In turn, in the system of sunflower oil, the increase in losses of polyphenols arising from addition of ferrous ions was not statistically significant (P > 0.05), probably due to the already very high levels of polyphenols degradation already in the system without iron.
Complex model systems
In the complex systems, the smallest degradation of GCE hydroxycinnamic acids took place in the protein and saccharose heated systems and amounted to 23 %, and the highest in the oil and starch system, which reached 47 %. Thus, the polyphenols losses in more or less complex systems reached levels similar to those of single substances, excluding sunflower oil, which, when heated alone with GCE, caused a very high level of polyphenol degradation, not observed in complex systems. However, the influence of sunflower oil heated with GCE, unfavorable for the stability of hydroxycinnamic acids, was likewise observed in the case of complex systems. Among complex systems, the losses of polyphenols were the highest in these containing sunflower oil and they amounted to 40–47 %. Only in the mentioned complex systems, the polyphenol degradation exceeded the 40 % level. The adverse influence of oil for the stability of hydroxycinnamic acids seems to be limited in the complex systems due to the presence of additional substances, which reduced the oil/hydroxycinnamic acids ratio and further physically limited the contact of GCE with the oil and its degradation products. Furthermore, in systems containing lipids, proteins, and polyphenols, the lipid radicals could partially react not with polyphenols but with proteins [27] and that might be the reason why the degradation of hydroxycinnamic acids from GCE in systems with oil and protein may be lower than in those containing only the oil and GCE (Table 2).
Changes of hydroxycinnamic acids in model systems upon heating
Among hydroxycinnamic acids, 5-O-CQA was the main component both in the initial GCE and in the heated model systems supplemented with GCE. In the green coffee extract, it constituted 30 % of polyphenols, and after heating the GCE in the model systems, its concentration increased to 40–50 % of the remaining hydroxycinnamic acids. During heating of caffeoylquinic acid, isomers transacylation of 3- and 4- isomers to 5-O-CQA isomer was observed, so its content in the heated product was in some systems even higher than in the starting unheated mixture [28]. The increase in the content of 5-O-CQA and its isomers may be also due to partial diesters hydrolysis. The content of them drastically decreased in heated systems of GCE and investigated substances. These changes are advantageous, due to the fact that the diesters are largely responsible for the bitter taste of coffee extracts [29–31]. Similar trends of hydroxycinnamic acids changes occur during roasting of coffee beans [6].
Relation between the degradation of polyphenols and antioxidative activity of model systems
The relation between the antioxidant activity marked in the studied systems by the method with DPPH˙ radical, presented in earlier reports, and the amount of maintained GCE polyphenols was analyzed [32, 33]. The test with radical was carried out by combining of aqueous or ethanolic (if contained oil [34]) solutions of four different concentrations of each system and methanolic solution of the radical. The absorbance of mixed solutions at 517 nm was measured. The antioxidant activity was calculated as percentage of reducing of the radical form, i.e., reducing of the absorbance. Next, a calibration curve of the dependence of concentration of the model system and antioxidant activity was obtained. On that basis, the concentration of the system, at which the quantity of radical form was reduced by 50 % (IC50), was calculated. Low IC50 values represent the high radical scavenging ability [35]. The cited research has shown that the supplementation of heated systems with GCE every time caused an increase in antioxidant activity. The results were presented in the IC50 system versus losses of hydroxycinnamic acids (Fig. 3). A negative trend between the degradation of polyphenols and IC50 was observed with a correlation coefficient at a level of −0.38. The determined trend therefore shows the increase in radical scavenging ability with the increasing degradation of hydroxycinnamic acids, although the trend is rather weak. This means that the polyphenols degradation products contribute to some extent to the increase in the antioxidant activity. A similar trend can be observed, for example, during coffee beans roasting, where the content of hydroxycinnamic acids drastically decreases but the antioxidant activity is maintained at the similar level and even may increase depending on the kind of bean and roasting conditions [35, 36]. Such relation is especially seen with the used method of antioxidant activity determination using DPPH˙, which is sensitive to both, polyphenols and the Maillard reaction product [35, 36].
Correlation of antioxidant activity determined using DPPH test [31] to hydroxycinnamic acids degradation. Values are mean ± SD, n = 6. Solid line systems with sunflower oil, open diamond- systems with starch, filled square systems with egg white protein, filled diamond systems with saccharose, 1 heated 0.5 h, 2 heated 1 h, 3 heated 1 h with Fe filled triangle complex system with: P egg white protein, S saccharose, ST starch, O sunflower oil
The negative trend was the most visible in the case of systems containing sunflower oil, where, despite the high level of degradation of hydroxycinnamic acids, a high antioxidant activity was found. This highly negatively correlated trend obtained in the case of systems with this component influenced a negative dependency calculated for all the examined model systems. Taking into account the relation between hydroxycinnamic acids degradation and radical scavenging capacity only among the systems with a single substance and GCE, it can be concluded that a decrease in the antioxidant activity along with polyphenols degradation was observed. This observation is in turn consistent with previous reports relating to polyphenols degradation, which showed a reduced ability to scavenge radicals by polyphenols thermal degradation products when compared to initial polyphenols [37]. Heating of GCE components with sunflower oil probably resulted in forming ingredients of high antioxidant activity. It should be noted that fresh oil was also characterized by high antioxidant activity (content of vitamin E—500 mg kg−1—manufacturers’ data), what could have an effect on subsequent results after heating with GCE. Oil heated alone significantly lost the ability to scavenge radicals, which was in turn well maintained in the system with GCE (work in progress). The next step in this research direction should be therefore answering the question whether the synergistic effect of tocopherols with hydroxycinnamic acids [38], or the compounds arising from the degradation of oil and GCE components heated together have major influence on the high antioxidant activity of such a system.
The obtained results in terms of relatively high antioxidant activity of heated systems confirmed the research of Charurin et al. [39]. In studies of unheated and heated model systems of arginine and saccharose optionally with chlorogenic acid, they showed that the addition of chlorogenic acid caused a visible increase in antioxidant activity of the systems, even higher after subjecting the systems to the heating. Heating of chlorogenic acid with inert material did not influence its antioxidant activity, which points out that the products of thermal degradation of chlorogenic acid also have a high antioxidant activity. Research on this topic was conducted by Andueza et al. [37], who demonstrated that among the products of caffeic acid oxidation are ethylcatechol, vinylcatechol, phenylindanes, and quinones, which in the later stage of oxidation form polymers with high antioxidant activity. Regarding the addition of iron ions, a deterioration of antioxidative properties was observed, coming from the reaction of hydroxycinnamic acids with iron. These results differ from earlier research on flavonoids [40], probably due to a lower number of hydroxyl groups in hydroxycinnamic acids, which in this case are blocked by the formed chelates.
When discussing the antioxidative activity of obtained model systems, one must also mention the contribution of caffeine to their antioxidant activity [41]. The concentrations of caffeine in the analyzed systems decreased by 1.5–10 % upon heating. The lowest level of degradation was observed in the system containing only protein, heated for 0.5 h, whereas the highest took place when the system also contained starch and sunflower oil. The level of degradation of caffeine was relatively low when compared to the degradation of polyphenols. No clear trend was established between the losses of caffeine and the type of system, heating time, or antioxidant activity. The correlation coefficient in this last case was equal to only r = 0.05. The influence of the presence of oil in the model systems on statistically higher degradation of this component was the only thing found.
Raw GCE has an intensely bitter flavor and cereal aroma. These GCE flavor features are felt intensively in food supplemented with this preparation. So its dosage to foods is often limited by organoleptic properties. However, if exposed to heat in complex food systems, its flavor and aroma change to caramel, toasted, nutty, etc., advantageously affecting the organoleptic properties of foods [42]. Therefore, if heating does not decrease drastically the antioxidant properties of food systems containing GCE and probably improves their organoleptic properties, this extract can be beneficially added to food prior to heating, despite the partial degradation of GCE polyphenols. The addition of GCE to food, which is processed thermally, like cake, may improve the health benefit of this product. Thus, the observations made in model systems were later used for preparing real foods in the form of 9 products, mostly confectionery and bakery products, supplemented with GCE, which, due to the large amount of data, are described in a separate publication.
Summary
Heating of hydroxycinnamic acids originating from green coffee bean in the systems with saccharose, starch, egg white protein, and sunflower oil causes a gradual degradation of these polyphenols. To the greatest extent, the degradative influence came from the presence of oil in the systems. Despite this, the addition of antioxidants in the form of hydroxycinnamic acids to heated foods containing sunflower oil can be beneficial due to their potentially high antioxidant activity. To design foods subjected to heat operations, which had to contain approximately 1 % of hydroxycinnamic acids after heating, the losses of polyphenols occurring during heat processing must be taken into account.
References
Cavin C, Marin-Kuan M, Langouët S, Bezençon C, Guignard G, Verguet C, Piguet D, Holzhäuser D, Cornaz R, Schilter B (2008) Induction of Nrf2-mediated cellular defenses and alteration of phase I activities as mechanisms of chemo protective effects of coffee in the liver. Food Chem Toxicol 46:1239–1248
Bichler J, Cavin C, Simic T, Chakraborty A, Ferk F, Hoelzl C, Schulte-Hermann R, Kundi M, Haidinger G, Angelis K, Knasmüller S (2007) Coffee consumption protects human lymphocytes against oxidative and 3-amino-1-methyl-5H-pyrido[4,3-b]indoleacetate (Trp-P-2) induced DNA-demage: results of an experimental study with human volunteers. Food Chem Toxicol 45:1428–1436
Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB (2004) Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med 140:1–8
Manach C, Scalbert A, Morand C, Rémésy C, Jiménaz J (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, Brighenti F (2003) Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr 133:2812–2819
Budryn G, Nebesny E, Podsędek A, Żyżelewicz D, Materska M, Jankowski S, Janda B (2009) Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and Maillard reaction products in coffee beans. Eur Food Res Technol 228:913–922
Nissen LR, Byrne DV, Bertelsen G, Skibsted RH (2004) The antioxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Sci 68:485–495
Nissen LR, Huynh-Ba T, Petersen MA, Bertelsen G, Skibsted LH (2002) Potential use of electron spin resonance spectroscopy for evaluating the oxidative status of potato flakes. Food Chem 79:387–394
Nissen LR, Månsson L, Bertelsen G, Huyanh-Ba T, Skibsed LH (2000) Protection of dehydrated chicken meat by natural antioxidants as evaluated by electron spin resonance spectrometry. J Agric Food Chem 48:5548–5556
Valencia I, O`Grady MN, Ansorena D, Astiasarán I, Kerry JP (2008) Enhancement of the nutritional status and quality of fresh pork sausages following the addition of linseed oil, fish oil and natural antioxidants. Meat Sci 80:1046–1054
Takenaka M, Sato N, Asakawa H, Wen X, Murata M, Homma S (2005) Characterization of a metal-chelating substance in coffee. Biosci Biotechnol Biochem 69:26–30
Raghavendra MP, Ramesh Kumar P, Prakash V (2007) Mechanism of inhibition of rice bran lipase by polyphenols: a case study with chloro genic acid and caffeic acid. J Food Sci 72:E412–E419
Anwar F, Jamil A, Iqbal S, Sheikh MA (2006) Antioxidant activity of various plant extracts under ambient and accelerated storage of sunflower oil. Grasas Aceites 57:189–197
Budryn G, Nebesny E, Żyżelewicz D (2011) Oxidative stability of lard and sunflower oil supplemented with coffee extracts under storage conditions. Grasas Aceites 62:155–161
Maat J, Rossi D, Babuchowski A, Beekmans F, Castenmiller J, Fenwick R, Haber J, Hogg T, Israelachwili D, Kettlitz B, Kohnke J, Lienemann K, Majou D, Petersen B, Schiefer G, Tomás-Barberán F (2005) European technology platform on food for life. The vision for 2020 and beyond. http://www.platformazywnosci.pl/pliki/BAT_Brochure_ETP.pdf. Accessed 30 Oct 2012
Crozier A, Jaganath IB, Clifford MN (2009) Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep 26:1001–1043
Harland BF (2000) Caffeine and nutrition. Nutrition 16:522–526
Williamson G, Holst B (2008) Dietary reference intake (DRI) value for dietary polyphenols: are we heading in the right direction? Brit J Nutr 99(Suppl 3):S55–S58
Glei M, Kirmse A, Habermann N, Persin C, Pool-Zobel BL (2006) Bread enriched with green coffee extract has chemo protective and anti genotoxic activities in human cells. Nutr Cancer 56:182–192
Suliburska J, Krejpcio Z, Kołaczyk N (2011) Evaluation of the content and the potential bioavailability of iron from fortified with iron and non-fortified food products. Acta Sci Pol Technol Aliment 10:233–243
Clifford MN (1985) Chlorogenic acids. In: Clarke RJ, Macrae R (eds) Coffee, vol 1. Chemistry, Elsevier Applied Science, London, pp 153–202
Prigent SVE, Voragen AGJ, Visser AJWG, Koningsveld GA, Gruppen H (2007) Covalent interactions between proteins and oxidation products of caffeoylquinic acid (chlorogenic acid). J Sci Food Agric 87:2502–2510
Prigent SVE, Voragen AGJ, Li F, Visser AJWG, Koningsveld GA, Gruppen H (2008) Covalent interactions between amino acid side chains and oxidation products of caffeoylquinic acid (chlorogenic acid). J Sci Food Agric 88:1748–1754
Prigent SVE, Gruppen H, Visser AJWG, Koningsveld GA, Jong GAH, Voragen AGJ (2003) Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J Agric Food Chem 51:5088–5095
Dupas CJ, Marsset-Baglieri AC, Ordonaut CS, Ducept FMG, Maillard MN (2006) Coffee antioxidant properties: effect of milk addition and processing conditions. J Food Sci 71:253–258
Hedegaard RV, Granby K, Frandsen H, Thygesen J, Skibsted LH (2008) Acrylamide in bread. Effect of prooxidants and antioxidants. Eur Food Res Technol 227:519–525
Viljanen K, Kivikari R, Heinonen M (2004) Protein-lipid interactions during liposome oxidation with added anthocyanin and other phenolic compounds. J Agric Food Chem 52:1104–1112835
Budryn G, Żyżelewicz D, Nebesny E, Oracz J, Krysiak W (2013) Influence of addition of green tea and green coffee extracts on the properties of fine yeast pastry fried products. Food Res Int 50:149–160
Frank O, Zehentbauer G, Hofmann T (2006) Bioresponse-guided decomposition of roast coffee beverage and identification of key bitter taste compounds. Eur Food Res Technol 222:492–508
Frank O, Blumberg S, Kunert C, Zehentbauer G, Hofmann T (2007) Structure determination and sensory analysis of bitter-tasting 4-vinylcatechol oligomers and their identification in roasted coffee by means of LC-MS/MS. J Agric Food Chem 55:1945–1954
Kreppenhofer S, Frank O, Hofmann T (2011) Identification of (furan-2-yl) methylated benzene diols and triols as a novel class of bitter compounds in roasted coffee. Food Chem 126:441–449
Budryn G, Nebesny E, Rachwał-Rosiak D (2012) Pepsin digestibility and antioxidant activity of egg white protein in model systems with green coffee extract. Int J Food Prop 40 (in press)
Nicoli MC, Anese M, Manzocco L, Lerici CR (1997) Antioxidant properties of coffee brews in relation to the roasting degree. Lebensm Wiss Technol 30:292–297
Ramadan MF (2013) Healthy blends of high linoleic sunflower oil with selected cold pressed oils: functionality, stability and antioxidative characteristics. Ind Crop Prod 43:65–72
Budryn G, Nebesny E (2008) Antioxidant properties of Arabica and Robusta coffee extracts prepared under different conditions. Deut Lebensm Rund 104:69–77
Perrone D, Farah A, Donangelo CM (2012) Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J Agric Food Chem 60:4265–4275
Andueza S, Manzocco L, De Pena MP, Cid C, Nicoli C (2009) Caffeic acid decomposition products: antioxidants or pro-oxidants? Food Res Int 42:51–55
Iglesias J, Pazos M, Andersen ML, Skibsted LH, Medina I (2009) Caffeic acid as antioxidant in fish muscle: mechanism of synergism with endogenous ascorbic acid and α-tocopherol. J Agric Food Chem 57:657–681
Charurin P, Ames JM, Castillo MD (2002) Antioxidant activity of coffee model system. J Agric Food Chem 50:3751–3756
Perron NR, Brumaghim JL (2009) A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys 53:75–100
Devasagayam TPA, Kamat JP, Mohan H, Kesavan PC (1996) Caffeine as an antioxidant: inhibition of lipids peroxidation induces by reactive oxygen species. Biochim Biophys Acta 1282:63–70
Jiang D, Peterson DG (2010) Role of hydroxycinnamic acids in the food flavor: a brief overview. Phytochem Rev 9:187–193
Acknowledgments
Authors are grateful for the financial support provided by Polish Ministry of Science and High Education (project No. NN 312 300137).
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Budryn, G., Nebesny, E. & Rachwał-Rosiak, D. Stability of hydroxycinnamic acids and caffeine from green coffee extracts after heating in food model systems. Eur Food Res Technol 236, 969–978 (2013). https://doi.org/10.1007/s00217-013-1956-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-1956-3