Abstract
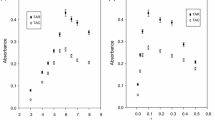
A cloud point extraction (CPE) method has been developed for the pre-concentration and simultaneous determination of lead [Pb(II)] and tin [Sn(II)] using Acridine Orange as complexing reagent and mediated by nonionic surfactant (Triton X-114) by flame atomic absorption spectrometry (FAAS). The main factors affecting analytical performance of CPE have been investigated in detail. The extracted surfactant-rich phase was diluted with (1.0 mol L−1) nitric acid in methanol, prior to subjecting FAAS. The calibration graphs obtained from Pb(II) and Sn(II) were linear in the concentration ranges of 5–1,000 and 10–5,000 μg L−1 with detection limits of 1.40 and 2.86 μg L−1, respectively. The relative standard deviations for 10 replicates containing 25 μg L−1 of Pb(II) and Sn(II) were 2.15 and 2.50 %, respectively. The analytical procedure was verified by the analysis of the standard reference materials NWTMDA-61.2 (water-trace elements) and NIST SRM 1548a (typical diet). The developed method has been applied to the simultaneous determination of total Pb and Sn in canned food samples including juices, tomato paste, corn, and green pea.





Similar content being viewed by others
References
Turker AR (2012) Sep Purif Rev 41:169–206
TS 266 (Turkish Standard) (2005) Water intended for human consumption (in Turkish), Ankara
WHO (2008) Guidelines for drinking water quality, volume 1, recommendation. World Health Organization, Geneva
Baysal A, Ozcan M, Akman S (2011) Food Chem Toxicol 49:1399–1403
Ulusoy S, Ulusoy HI, Akçay M, Gürkan R (2012) Food Chem 134:419–426
Zhu X, Zhu X, Wang B (2006) J Anal At Spectrom 21:69–73
Huang X, Zhang W, Han S, Wang X (1997) Talanta 44:817–822
Silva EL, Roldan PS, Giné MF (2009) J Hazard Mater 171(1–3):1133–1138
Ortega C, Cerutti S, Roberto AO (2004) J Pharmaceut Biomed 36:721–727
Manzoori JL, Bavili-Tabrizi A (2002) Anal Chim Acta 470:215–221
Lemos VA, David GT (2010) Microchem J 94(1):42–47
Ulusoy HI, Gürkan R, Aksoy U, Akçay M (2011) Microchem J 99:76–81
Ulusoy HI, Gürkan R, Ulusoy S (2012) Talanta 88:516–523
Ulusoy HI, Gürkan R, Demir O, Ulusoy S (2012) Food Anal Methods 5:454–463
Sahin CA, Efecınar M, Satıroglu N (2010) J Hazard Mater 176:672–677
Beceiro-González E, Guimaraes A, Alpendurad MF (2009) J Chromatogr A 1216(29):5563–5569
Soylak M, Tuzen M (2008) J Hazard Mater 152:656–661
Soylak M, Yilmaz E (2011) Desalination 275:297–301
Bakircioglu Y, Bakircioglu D, Akman S (2010) J Hazard Mater 178:1015–1020
Ghaedi M, Shokrollahi A, Niknam K, Niknama E, Najibi A, Soylak M (2009) J Hazard Mater 168:1022–1027
Falcone RD, Correa NM, Biasutti MA, Silber JJ (2002) Langmuir 18:2039–2047
Schuleman SG, Naik DV, Capomacchia AC, Roy TJ (1975) Pharm Sci 64:982–986
Ghosh AK, Samanta A, Bandyopadhyay P (2011) J Phys Chem B 115:11823–11830
Ghosh AK, Samanta A, Bandyopadhyay P (2011) Chem Phys Lett 507:162–167
Baes CF, Messmer RE (1976) The hydrolysis constants occasions. Wiley-Interscience, New York
Luo YJ, Shen HX (1999) Anal Commun 36:135–137
Madej K (2009) Trends Anal Chem 28:436–446
Evdokimov E, Wandruska R (1998) Anal Lett 31:2289–2298
Komaromy-Hiller G, Calkins N, Wandruszka R (1996) Langmuir 12(4):916–920
Gholivanda MB, Babakhaniana A, Rafieeb E (2008) Talanta 76:503–508
Madrakian T, Ghazizadeh F (2009) J Braz Chem Soc 20(8):1535–1540
Tavallali H, Asrari E, Attaran AM, Tabandeh M (2010) Int J Chem Tech Res 2(3):1731–1737
Han H, Yayun X, Zhang C (2011) Commun Soil Sci Plan 42:1739–1751
Shiri S, Delpisheh A, Haeri A, Poornajaf A, Golzadeh B, Shiri S (2011) Anal Chem Insights 6:15–20
Shemirani F, Abkenar SD, Khatouni A (2004) Bull Korean Chem Soc 25(8):1133–1136
Citak D, Tuzen M (2010) Food Chem Toxicol 48:1399–1403
Andrade FP, Nascentes CC, Costa LM (2009) J Braz Chem Soc 20(8):1460–1466
Bakircioglu D (2012) Environ Sci Pollut Res 19(6):2428–2437
Acknowledgments
This study has been supported by Cumhuriyet University Scientific Research Projects Commission as the research projects with the F-367 code.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulusoy, H.İ., Aksoy, Ü. & Akçay, M. Simultaneous pre-concentration of Pb and Sn in food samples and determination by atomic absorption spectrometry. Eur Food Res Technol 236, 725–733 (2013). https://doi.org/10.1007/s00217-013-1929-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-1929-6