Abstract
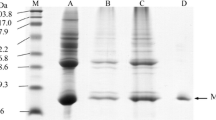
The influences of Fenton’s reactants (H2O2 and FeCl2) and aldehydes (hexanal and hexenal) on changes of oxymyoglobin and metmyoglobin from Eastern little tuna (Euthynnus affinis) dark muscle were studied. In the presence of H2O2, both oxymyoglobin and metmyoglobin were rapidly oxidized into ferrylmyoglobin based on spectra patterns. In the presence of Fe2+ and/or H2O2, the changes in fluorescent intensity of myoglobin were noticeable, but there were no changes in aggregation ratio. Release of non-heme iron from myoglobin was mainly governed by H2O2. When aldehydes were incorporated, the oxidation of oxymyoglobin and conformational changes of globin were more pronounced. No release of non-heme iron was noticeable, suggesting the stability of heme moiety toward aldehydes. Hexenal had a great impact on cross-linking of oxymyoglobin and metmyoglobin via covalent modification. Alteration of myoglobin redox state might be enhanced by conformational changes of globin induced by both Fenton’s reactants and aldehydes.





Similar content being viewed by others
References
O’Grady MN, Monahan FJ, Brunton NP (2001) J Food Sci 66:386–392
Richards MP, Dettmann MA, Grunwald EW (2005) J Agric Food Chem 53:10231–10238
Chan WKM, Faustman C, Yin M, Decker EA (1997) Meat Sci 46:181–190
Baron CP, Skibsted LH, Andersen HJ (2000) Free Radic Biol Med 28:549–558
Davies MJ (1991) Biochim Biophys Acta 1077:86–90
Robinson S, Dang T, Dringen R, Bishop G (2009) Redox Rep 14:228–235
Vuletich JL, Osawa Y, Aviram M (2000) Biochem Biophys Res Commun 269:647–651
Baron CP, Andersen HJ (2002) J Agric Food Chem 50:3887–3897
Faustman C, Liebler DC, McClure TD, Sun Q (1999) J Agric Food Chem 47:3140–3144
Lynch MP, Faustman C (2000) J Agric Food Chem 48:600–604
Lee S, Joo ST, Alderton AL, Hill DW, Faustman C (2003) J Food Sci 68:1664–1668
Lee S, Phillips AL, Liebler DC, Faustman C (2003) Meat Sci 63:241–247
Fisheries Foreign Affairs Division (2007) Statistics on Fishery Production 2007, Ministry of Agriculture and Co-operatives. http://www.fisheries.go.th/it-stat/data_2550/yearbook2007%282550%29/yearbook2007%2038%20T2.1.pdf. Accessed 19 March 2010
Thiansilakul Y, Benjakul S, Richards MP (2011) Food Chem 124:254–261
Tang J, Faustman C, Hoagland TA (2004) J Food Sci 69:717–720
Swatland HJ (1989) Can Inst Food Sci Technol J 22:390–402
Chanthai S, Ogawa M, Tamiya T, Tsuchiya T (1996) Fish Sci 62:927–932
Chow CJ, Ochiai Y, Watabe S (2004) J Food Biochem 28:123–134
Schricker BR, Miller DD, Stouffer JR (1982) J Food Sci 47:740–743
Laemmli UK (1970) Nature 227:680–685
Steel RGD, Torrie JH (1980) Principles and procedures of statistics; a biometrical approach. McGraw-Hill Book, New York
Cooper CE, Jurd M, Nicholls P, Wankasi MM, Svistunenko DA, Reeder BJ, Wilson MT (2005) Dalton Trans 21:3483–3488
Romero F, Ordonez I, Arduini A, Cadenas E (1992) J Biol Chem 267:1680–1688
Prasad M, Engelman R, Jones R, Das D (1989) Biochem J 263:731–736
DeGray JA, Gunther MR, Tschirret-Guth R, de Montellano PRO, Mason RP (1997) J Biol Chem 272:2359–2362
Bontidean I, Berggren C, Johansson G, Csoregi E, Mattiasson B, Lloyd JR, Jakeman KJ, Brown NL (1998) Anal Chem 70:4162–4169
Gajewski E, Dizdaroglu M (1990) Biochemistry 29:977–980
Uchida K, Kato Y, Kawakishi S (1990) Biochem Biophys Res Commun 169:265–271
Chi EY, Krishnan S, Randolph TW, Carpenter JF (2003) Pharm Res 20:1325–1336
Livingston DJ, Brown WD (1981) Food Technol 35:238–252
Maheswarappa NB, Faustman C, Tatiyaborworntham N, Yin S, Ramanathan R, Mancini RA (2009) J Agric Food Chem 57:8668–8676
Libondi T, Ragone R, Vincenti D, Stiuso P, Auricchio G, Colonna G (1994) Int J Pept Protein Res 44:342–347
Acknowledgments
This research was supported by the Thailand Research Fund under the Royal Golden Jubilee PhD Program to Yaowapa Thiansilakul (PHD/0101/2550) and TRF senior research scholar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thiansilakul, Y., Benjakul, S. & Richards, M.P. The effect of Fenton’s reactants and aldehydes on the changes of myoglobin from Eastern little tuna (Euthynnus affinis) dark muscle. Eur Food Res Technol 232, 221–230 (2011). https://doi.org/10.1007/s00217-010-1370-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1370-z