Abstract
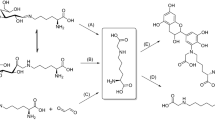
A previously uncharacterized glycation compound was isolated from a reaction mixture of N-α-Boc-lysine and 3-deoxypentosone by semi-preparative ion-exchange chromatography and was identified by nuclear magnetic resonance (NMR) spectroscopy as 6-(2-formyl-1-pyrrolyl)-l-norleucine (formyline). 3-Deoxypentosone and pentoses like ribose, arabinose, or xylose were identified as the predominant precursors of the new glycation compound, but formyline can also be formed from lysine and degradation products of disaccharides and glucuronic acid. The Amadori products lactuloselysine and ribuloselysine, which were synthesized by improved methods and characterized by NMR spectroscopy, were shown not to form formyline when heated in dry state without lysine added, indicating that reactions between the lysine side chain and dicarbonyl compounds are the main pathways for formyline formation rather than transformation of lysine containing Amadori products. Finally, it was shown by HPLC analysis after enzymatic digestion that peptide-bound formyline can be formed during incubation of casein and 3-deoxypentosone under conditions comparable to food processing.







Similar content being viewed by others
References
Ledl F, Schleicher E (1990) Angew Chem 102:597–626
Henle T (2005) Amino Acids 29:313–322
Hodge JE (1953) J Agric Food Chem 1:928–943
Yaylayan VA, Huyghues-Despointes A (1994) Crit Rev Food Sci 34:321–369
Hollnagel A, Kroh LW (2002) J Agric Food Chem 50:1659–1664
Mavric E, Henle T (2006) Eur Food Res Technol 223:803–810
Thornalley PJ, Battah S, Ahmed N, Karachalias N, Agalou S, Babaei-Jadidi R, Dawnay A (2003) Biochem J 375:581–592
Henle T, Miyata T (2003) Adv Ren Replace Ther 10:321–331
Linden T, Cohen A, Deppisch R, Kjellstrand P, Wieslander A (2002) Kidney Int 62:697–703
Tarr HLA (1953) Nature 171:344–345
Munanairi A, O’Banion SK, Gamble R, Breuer E, Harris AW, Sandwick RK (2007) Carbohydr Res 342:2575–2592
Cuzzoni MT, Stoppini G, Gazzani G, Mazza P (1988) Food Chem Toxic 26:815–822
Vagnarelli P, De Sario A, Cuzzoni MT, Mazza P, De Carli L (1991) J Agric Food Chem 39:2237–2239
Fortpied J, Gemayel R, Stroobant V, van Schaftingen E (2005) Biochem J 388:795–802
Sopio R, Lederer MO (1995) Z Lebensm Unters Forsch 201:381–386
Mavric E, Kumpf Y, Schuster K, Kappenstein O, Scheller D, Henle T (2004) Eur Food Res Technol 218:213–218
Sell DR, Monnier VM (1989) J Biol Chem 264:21597–21602
Henle T, Schwarzenbolz U, Klostermeyer H (1997) Z Lebensm Unters Forsch 204:95–98
Kato H (1967) Agric Biol Chem 31:1086–1090
Tressl R, Kersten E, Rewicki D (1993) J Agric Food Chem 41:2125–2130
Pachmayr O, Ledl F, Severin T (1986) Z Lebensm Unters Forsch 182:294–297
Argirov OK, Lin B, Ortwerth BJ (2004) J Biol Chem 279:6487–6495
Tressl R, Wondrak GT, Krüger RP, Rewicki D (1998) J Agric Food Chem 46:104–110
Henle T, Bachmann A (1996) Z Lebensm Unters Forsch 202:72–74
Zamora R, Alaiz M, Hidalgo FJ (1999) J Agric Food Chem 47:1942–1947
Henle T, Walter AW, Klostermeyer H (1994) In: Labuza TP (ed) Maillard reactions in food, chemistry, and health. The Royal Society of Chemistry, Cambridge, pp 195–200
Hellwig M, Geißler S, Peto A, Knütter I, Brandsch M, Henle T (2009) J Agric Food Chem 57:6474–6480
Siegl T (2003) PhD Thesis, TU Dresden
Krause R, Knoll K, Henle T (2003) Eur Food Res Technol 216:277–283
Vinale F, Monti SM, Panunzi B, Fogliano V (1999) J Agric Food Chem 47:4700–4706
Henle T, Walter H, Klostermeyer H (1991) Z Lebensm Unters Forsch 113:119–122
Chobert JM, Gaudin JC, Dalgalarrondo M, Haertlé T (2006) Biotechnol Adv 24:629–632
Rockland LB (1960) Anal Chem 32:1375–1376
Wei C, Pohorille A (2009) J Am Chem Soc 131:10237–10245
Horvat Š, Roščič M, Lemieux C, Nguyen TMD, Schiller PW (2007) Chem Biol Drug Des 70:30–39
Weenen H, van der Veen JGM, van der Linde LM, van Duynhoven J, Groenewegen A (1998) In: O’Brien J (ed) The Maillard reaction in foods and medicine. The Royal Society of Chemistry, Cambridge, pp 57–64
Schwarzenbolz U, Mende S, Henle T (2008) Ann N Y Acad Sci 1126:248–252
Acknowledgments
We thank Dr. Uwe Schwarzenbolz, Institute of Food Chemistry, for the acquisition of the mass spectra. We are grateful to the members of the Institute of Organic Chemistry, namely Dr. Margit Gruner and Anett Rudolph, for recording the NMR spectra and Anke Peritz for performing the elemental analyses. This work was supported by a research grant of the Deutsche Forschungsgemeinschaft (HE 2306/9-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hellwig, M., Henle, T. Formyline, a new glycation compound from the reaction of lysine and 3-deoxypentosone. Eur Food Res Technol 230, 903–914 (2010). https://doi.org/10.1007/s00217-010-1237-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1237-3