Abstract
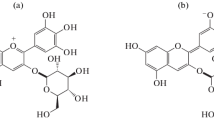
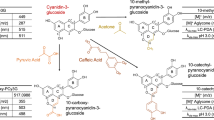
The objective of this study was to evaluate the influence of the degree of polymerisation of flavanols in their ability to act as anthocyanin copigments. With this aim, ethyl-bridged catechin derivatives were produced by reaction between catechin and acetaldehyde. Further fractionation in Toyopearl HW-40 allowed the separation of five fractions of increasing molecular size containing mixtures of compounds in the range from monomers to hexamers as established by LC-MS. The copigmentation assays were carried out in citrate-phosphate buffer solutions in 12% ethanol at pH 3.6, using malvidin 3-O-glucoside as pigment and increasing concentrations of the different catechin fractions to obtain copigment to pigment ratios ranging 0:1–20:1. Copigmentation was assessed from the modifications in the visible spectra of the solutions; chromatic analysis in the CIELAB colour space was used to characterise the copigmentation effect. It was found that all the compounds assayed were able to interact with the anthocyanin and induced changes in the colour of the solutions. The greatest effect was induced by oligomers containing 2–3 elementary catechin units, whereas monomers were the poorest anthocyanin copigments among the compounds checked. An interesting observation was that the formation of the copigmentation complexes was not and immediate process, but it required some time to attain the equilibrium, suggesting that the phenomenon was controlled thermodynamically rather than kinetically.




Similar content being viewed by others
References
Wilderandt HL, Singleton VL (1974) Am J Enol Vitic 25:119–126
Fulcrand H, Doco T, Es-Safi NE, Cheynier V, Moutounet M (1996) J Chromatogr A 752:85–91
Saucier C, Bourgeois G, Vitry C, Roux D, Glories Y (1997) J Agric Food Chem 45:1045–1049
Timberlake CF, Bridle P (1976) Am J Enol Vitic 27:97–105
Bakker J, Picinelli A, Bridle P (1993) Vitis 32:111–118
Rivas-Gonzalo J, Bravo-Haro S, Santos-Buelga C (1995) J Agric Food Chem 43:1444–1449
Atanasova V, Fulcrand H, Le-Guerneve C, Cheynier W, Moutounet M (2002) Tetrahedron Lett 43:6151–6153
Noble AC (1990) Bitterness and astringency in wine. In: Rouseff R (Ed) Bitterness in foods, beverages. Elsevier, Amsterdam, pp 145–158
Vidal S, Francis L, Noble A, Kwiatkowski M, Cheynier V, Waters E (2004) Anal Chim Acta 513:57–65
Saucier C, Guerra C, Pianet I., Laguerre M, Glories Y (1997) Phytochemistry 46:229–234
Somers TC, Wescombe LG (1987) Vitis 26:27–36
Mazza G, Brouillard R (1990) Phytochemistry 29:1097–1102
Asen S, Stewart RN, Norris KH (1972) Phytochemistry 11:1139–1145
Brouillard R, Wigand MC, Dangles O, Cheminat A (1991) J Chem Soc Perkin Trans 2:1235–1241
Boulton R (2001) Am J Enol Vitic 52:67–87
Brouillard R, Dangles O (1994) Food Chem 51:365–371
Liao H, Cai Y, Haslam E (1992) J Sci Food Agric 59:299–305
Gomez-Miguez M, Gonzalez-Manzano S, Escribano-Bailon MT, Heredia FJ, Santos-Buelga C (2006) J Agric Food Chem 54:5422–5429
Berke B, de Freitas VAP (2005) Food Chem 90:453–460
Mirabel M, Saucier C, Guerra C, Glories Y (1999) Am J Enol Vitic 50:211–218
Malien-Aubert C, Dangles O, Amiot MJ (2002) J Agric Food Chem 50:3299–3305
Mateus N, de Freitas V (2001) J Agric Food Chem 49:5217–5222
Heredia FJ, Álvarez C, González-Miret ML, Ramírez, A (2004) CromaLab®, Registro General de la Propiedad Intelectual SE-1052–04, Sevilla, Spain
CIE 15:2004 (2004) Technical report colorimetry, 3rd edn. CIE Central Bureau, ISBN 3 901 906 33 9
Es-Safi NE, Fulcrand H, Cheynier V, Moutounet M (1999) J Agric Food Chem 47:2088–2095
Gonzalez-Manzano S, Santos-Buelga C, Perez-Alonso JJ, Rivas-Gonzalo JC, Escribano-Bailon MT (2006) J Agric Food Chem 54:4326–4332
Cheynier V, Doco T, Fulcrand H, Guyot S, Le Roux E, Souquet JM, Rigaud J, Moutounet M (1997) Analusis 25:M32–M37
Escribano-Bailon T, Alvarez-Garcia M, Rivas-Gonzalo JC, Heredia FJ, Santos-Buelga C (2001) J Agric Food Chem 49:1213–1217
Duenas M, Salas E, Cheynier V, Dangles O, Fulcrand H (2006) J Agric Food Chem 54:189–196
Vivar-Quintana AM, Santos-Buelga C, Rivas-Gonzalo JC (2002) Analy Chim Acta 458:147–155
Martínez JA, Melgosa M, Pérez MM, Hita E, Negueruela AI (2001) Food Sci Technol Int 7:439–444
Acknowledgements
Financial support received from INIA (Grant ref. VIN03-043-C3), CICYT (Grant ref. AGL2005-07245-C03-01) and Ministerio de Educación y Ciencia (mobility Grant to author SGM, ref BES-2003-0816) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Manzano, S., Mateus, N., de Freitas, V. et al. Influence of the degree of polymerisation in the ability of catechins to act as anthocyanin copigments. Eur Food Res Technol 227, 83–92 (2008). https://doi.org/10.1007/s00217-007-0696-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0696-7