Abstract
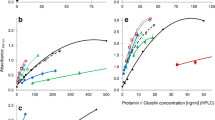
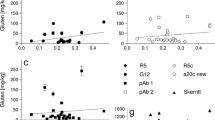
Immunoassays are the most commonly used quantitative techniques to determine the gliadin content of food aimed at coeliac patients. Though the minimal amount of gliadins inducing the typical histopathological changes at the intestinal mucosa in coeliacs is still a matter of debate, current research is focussed on the development of methods having higher sensitivities. One of the main drawbacks in gliadin analysis is the low efficiency of the conventional extraction procedure using 60% ethanol. The use of reducing (2-mercaptoethanol) and denaturing (guanidinium chloride) agents has been recommended to improve the extraction efficiency. Owing to the well-known effects of these agents on native conformation of proteins, and their widely reported interference on the antigen/antibody interaction in other systems, we assessed whether gliadin detection by immunoassays is affected by the presence of those agents. Using two ELISA formats with a panel of polyclonal and monoclonal antibodies, we found that recognition by specific antibodies of partially or totally denatured gliadins is severely impaired. The magnitude of the interference depends on the antibodies used and the ELISA format. The impact of such interference was analysed for each step of the immunoassays. 2-mercaptoethanol had a stronger effect than guanidinium chloride, and the antigen became almost undetectable for some assays when both reagents were used in combination. Remarkably, since quantitative results are obtained by comparison with a calibration curve using a native antigen, there is no equivalence between the antigen/antibody interaction occurring in the sample and that in the standard gliadin, leading to underestimation of the actual gliadin content. Therefore, we suggest that not only the effects of reducing and denaturing agents on the antigen during the extraction procedure, but also the effects of residual amounts of these agents on the antigen/antibody interaction should be considered when a quantitative immunoassay is performed.







Similar content being viewed by others
Abbreviations
- 2-ME:
-
2-Mercaptoethanol
- GuHCl:
-
Guanidinium chloride
- mAb:
-
Monoclonal antibody
References
Koning F (2005) Celiac disease: caught between a rock and a hard place. Gastroenterol 129:1294–1301
Londei M, Ciacci C, Ricciardelli I, Vacca L, Quaratino S, Maiuri L (2005) Gliadin as a stimulator of innate responses in celiac disease. Mol Immunol 42(8):913–918
Brandtzaeg P (2006) The changing immunological paradigm in coeliac disease. Immunol Lett 105(2):127–139
Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, Macneil J, Mack D, Patel D, Moher D (2005) The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterol 128(4 Suppl 1):S57–S67
Sollid LM, Khosla C (2005) Future therapeutic options for celiac disease. Nat Clin Pract Gastroenterol Hepatol 2(3):140–147
Ciclitira PJ, Ellis HJ, Lundin KE (2005) Gluten-free diet—what is toxic?. Best Pract Res Clin Gastroenterol 19(3):359–371
Rashid M, Cranney A, Zarkadas M, Graham ID, Switzer C, Case S, Molloy M, Warren RE, Burrows V, Butzner JD (2005) Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics 116(6):754–759
Abdulkarim A, Burgat L, See J, Murray JA (2002) Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol 97(8):2016–2021
Johnston SD, Rodgers C, Watson RG (2004) Quality of life in screen-detected and typical coeliac disease and the effect of excluding dietary gluten. Eur J Gastroenterol Hepatol 16(12):1281–1286
Zarkadas M, Cranney A, Case S, Molloy M, Switzer C, Graham ID, Butzner JD, Rashid M, Warren RE, Burrows V (2006) The impact of a gluten-free diet on adults with coeliac disease: results of a national survey. J Hum Nutr Diet 19(1):41–49
Kupper C (2005) Dietary guidelines and implementation for celiac disease. Gastroenterology 128(4 Suppl 1):S121–S127
Hopman EG, le Cessie S, von Blomberg BM, Mearin ML (2006) Nutritional management of the gluten-free diet in young people with celiac disease in The Netherlands. J Pediatr Gastroenterol Nutr 43(1):102–108
Case S (2005) The gluten-free diet: how to provide effective education and resources. Gastroenterology 128(4 Suppl 1):S128–S134
Hischenhuber C, Crevel R, Jarry B, Makis M, Moneret-Vautrin DA, Romano A, Troncote R, Ward R (2006) Safe amounts of gluten for patients with wheat allergy or coeliac disease. Alim Pharm Ther 23:559–575
Collin P, Thorell L, Kaukinen K, Maki M (2004) The safe threshold for gluten contamination in gluten-free products. Can trace amounts be accepted in the treatment of coeliac disease? Aliment Pharmacol Ther 19(12):1277–1283
Catassi C, Fabiani E, Iacono G, D’ Agate C, Francavilla R, Biagi F,Volta U, Accomando S, Picarelli A, De Vitis I, Pianelli G, Gesuita R,Carle F, Mandolesi A, Bearzi I, Fasano A (2007) A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 85(1):160–166.
Shewry PR, Tatham AS (1990) The prolamin storage proteins of cereal seeds: structure and evolution. Biochem J 267:1–12
Howdle PD, Ciclitira PJ, Simpson FG, Losowsky MS (1984) Are all gliadins toxic in coeliac disease? An in vitro study of alpha, beta, gamma, and w gliadins. Scand J Gastroenterol 19(1):41–47
Sturgess RP, Hooper LB, Spencer J, Hung CH, Nelufer JM, Ciclitira PJ (1992) Effects of interferon-gamma and tumor necrosis factor-alpha on epithelial HLA class-II expression on jejunal mucosal biopsy specimens cultured in vitro. Scand J Gastroenterol 27(11):907–911
Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P (1995) Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut 37(6):766–776
Molberg O, Mcadam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 4(6):713–717
Tollefsen S, Arentz-Hansen H, Fleckenstein B, Molberg O, Raki M, Kwok WW, Jung G, Lundin KE, Sollid LM (2006) HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J Clin Invest 116(8):2226–2236
Dewar DH, Amato M, Ellis HJ, Pollock EL, Gonzalez-Cinca N, Wieser H, Ciclitira PJ (2006) The toxicity of high molecular weight glutenin subunits of wheat to patients with coeliac disease. Eur J Gastroenterol Hepatol 18(5):483–491
Wieser H (1998) Investigations on the extractability of gluten proteins wheat bread in comparison with flour Z. Lebensm Unter Forshc A 207:128–132
Wieser H, Antes S, Seilmeier W (1998) Quantitative determination of gluten protein types in wheat flour by reversed-phase high performance liquid chromatography. Cereal Chem 75(5):644–650
Freedman AR, Galfre G, Gal E, Ellis HJ, Ciclitira PJ (1987) Monoclonal antibody ELISA to quantitate wheat gliadin contamination of gluten-free foods. J Immunol Meth 98(1):123–127
Chirdo FG, Añón MC, Fossati CA (1995) Optimization of a competitive ELISA for quantification of prolamins in food. Food Agric Immunol 7(4):333–343
Ellis J, Rosen-Bronson S, O´Reilly N, Ciclitira PJ (1998) Measurement of gluten using a monoclonal antibody to a coeliac toxic peptide of A-gliadin. Gut 43:190–195
Ferranti P, Mamone G, Melck D, Tafuro M, Picarriello G, Addeo F (2002) Combined mass spectrometric and immunological strategies for detection of gliadins in wheat varieties from Europe. In: Proceeding of the 16th Meeting of the European Working Group in Prolamin analysis and Toxicity, pp 29–36
Henterich N, Osman A, Mendez E, Mothes T (2003) Assay of gliadin by real-time immuno-polymerase chain reaction. Nahrung/Food 5:343–346
Valdés I, García E, Llorente M, Méndez E (2003) Innovative approach to low level gluten determination in foods using a novel sandwich ELISA protocol. Eur J Gastroenterol Hepatol 15(5):465–474
Spaenij-Dekkin EHA, Kooy-Winkelaar EMC, Nieuwenhuizen WF, Drijfhout JW, Koing F (2004) A novel and sensitive method for the detection of T cell stimulatory epitopes of α/β and γ-gliadin. Gut 53:1267–1273
Mendez E, Vela C, Immer U, Janssen FW (2005) Report of a collaborative trial to investigate the performance of the R5 enzyme linked immunoassay to determine gliadin in gluten-free food. Eur J Gastroenterol Hepatol 17(10):1053–1063
Kahlenberg F, Sanchez D, Lachmann I, Tuckova L, Tlaskalova H, Mendez E, Mothes T (2006) Monoclonal antibody R5 for detection of putatively celiac-toxic gliadin peptides. Eur Food Res Technol 222:78–82
Garcia E, Llorente M, Hernando A, Kieffer R, Wieser H, Mendez E (2005) Development of a general procedure for complete extraction of gliadins for heat processed and unheated foods. Eur J Gastroenterol Hepatol 7:529–539
Margheritis AI, Doña V, Fossati CA, Chirdo FG (2003) Effect of reducing agents on the immunochemical detection of gliadin. In: Stern M (ed) Proceeding of 17th meeting of the European working group on prolamin analysis and toxicity, 2–5 October, Stockholm, Sweden. 2003. Zwickau Germany: Verlag Wissenschaftliche Scripten 2004, pp 51–58
Doña V, Fossati CA, Chirdo FG (2003). Interference of denaturing and reducing agents on gliadin/antibody interaction. In: Stern M (ed) Proceeding of the 18th meeting of the European working group on prolamin analysis and toxicity, 2–5 October 2003, Stockholm, Sweden. 2003. Zwickau Germany: Verlag Wissenschaftliche Scripten 2004, pp 51–58
Chirdo FG, Añón MC, Fossati CA (1998) Development of high sensitive enzyme immunoassays for gliadin quantification using the streptavidin–biotin amplification system. Food Agric Immunol 10(2):143–155
Yang HJ, Tsou CL (1995) Inactivation during denaturation of ribonuclease A by guanidinium chloride is accompanied by unfolding at the active site. Biochem J 305(Pt 2):379–384
Wang GF, Cao ZF, Zhou HM, Zhao YF (2000) Comparison of inactivation and unfolding of methanol dehydrogenase during denaturation in guanidine hydrochloride and urea. Int J Biochem Cell Biol 32:873–878
Wang XD, Luo ZQ, Zhou JM, Tsou CL (1997) Perturbation of the antigen-binding site and staphylococcal protein A-binding site of IgG before significant changes in global conformation during denaturation: an equilibrium study. Biochem J 325:707–710
Singh RR, Chang JY (2004) Investigating conformational stability of bovine pancreatic phospholipase A2: a novel concept in evaluating the contribution of the “native framework” of disulphides to the global conformational stability of proteins. Biochem J 377:685–692
Author information
Authors and Affiliations
Corresponding author
Additional information
V.V. D is postgraduate fellow of CONICET. C.A.F. and F.G.Ch. are members of the Researcher Career of CONICET. This study was supported by the Grant PICT 9800 from ANPCyT and a Grant from the Comisión Investigaciones Científicas de la Provincia de Buenos Aires.
Rights and permissions
About this article
Cite this article
Doña, V.V., Fossati, C.A. & Chirdo, F.G. Interference of denaturing and reducing agents on the antigen/antibody interaction. Impact on the performance of quantitative immunoassays in gliadin analysis. Eur Food Res Technol 226, 591–602 (2008). https://doi.org/10.1007/s00217-007-0597-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0597-9