Abstract
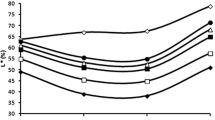
Copigmentation processes have been indicated to be crucial to stabilise coloured forms of the anthocyanins and explain colour expression in young red wines. Several studies exist about copigmentation between anthocyanins and different phenolics in model solutions, but little information is available about interactions among anthocyanins themselves. In this work, the process of self-association has been investigated in wine-like model solutions containing different grape anthocyanins (the 3-glucosides of malvidin, delphinidin and peonidin). The results obtained confirmed the existence of anthocyanin self-association and its influence on the apparent hydration constant of the anthocyanins with subsequent modification in the colour of the solutions. It was observed that the greater the degree of methoxylation of the anthocyanin B-ring the greater was the magnitude of the self-association. Colour analyses in the CIELAB space showed that self-association produces changes, which are more important in quantitative parameters (chroma, C *ab and lightness, L *) than in qualitative ones (hue, h ab). Self-association leads to an increase in C *ab , indicating a more intense colour of the solutions, and to a decrease in the psychometric index L *, meaning that a darkening is produced. The effects on the colour were more pronounced with the passage of time of storage of the solutions.





Similar content being viewed by others
References
Boulton RBA (1996) Presented at the forty-seventh annual meeting of the American society for enology and viticulture. Reno, NV
Asen S, Stewart RN, Norris KH (1972) Phytochemistry 11:1139–1144
Brouillard R, Wigand MC, Dangles O, Cheminat A (1991) J Chem Soc Perkin Trans 2:1235–1241
Liao H, Cai Y, Haslam E (1992) J Sci Food Agric 59:299–305
Davies AJ, Mazza G (1993) J Agric Food Chem 41:716–720
Goto T, Kondo T (1991) Angew Chem Int Ed Engl 30:17–33
Hoshino T, Matsumoto U, Goto T (1980) Phytochemistry 19:663–667
Hoshino T, Matsumoto U, Goto T (1981) Phytochemistry 20:1971–1976
Hoshino T, Matsumoto U, Harada N, Goto T (1981) Tetrahedron Lett 22:3621–3624
Hoshino T, Matsumoto U, Goto T, Harada N (1982) Tetrahedron Lett 23:433–436
Hoshino T, Matsumoto U (1980) Tetrahedron Lett 21:1751–1754
Timberlake CF (1980) Food Chem 5:69–80
Miniati E, Damiani P, Mazza G (1992) Ital J Food Sci 4:109–116
Houbiers C, Lima JC, Maçanita AL, Santos H (1998) J Phys Chem B 102:3578–3585
Boulton R (2001) Am J Enol Vitic 52:67–84
Hoshino T (1991) Phytochemistry 30:2049–2055
Somers TC, Evans ME (1979) J Sci Food Agric 30:623–633
Heredia FJ, Francia-Aricha EM, Rivas-Gonzalo JC, Vicario IM, Santos Buelga C (1998) Food Chem 63:491–498
Dueñas M, Salas E, Cheynier V, Dangles O, Fulcrand H (2006) J Agric Food Chem 54:189–196
Dangles O, Saito N, Brouillard R (1993) J Am Chem Soc 115:3125–3132
Alcalde-Eon C, Escribano-Bailón MT, Santos-Buelga C, Rivas-Gonzalo J (2004) Anal Chim Acta 513:305–318
Heredia FJ, Álvarez C, González-Miret ML, Ramírez A (2004) Registro General de la Propiedad Intelectual SE-1052-04 Sevilla, Spain
CIE 15:2004, Technical report colorimetry, 3rd edn. CIE Central Bureau, 2004. ISBN 3 901 906 33 9
Figueiredo P, Elhabiri M, Toki K, Saito N, Dangles O, Brouillard R (1995) Phytochemistry 41:301–308
Markakis P (1982) Stability of anthocyanins in foods. In: Markakis P (ed) Anthocyanins as food colors, 1st edn. Academic, New York, 163–178
Furtado P, Figueiredo P, Chaves-das-Neves H, Pina FJ (1993) Photochem Photobiol A 78:113–118
Eiro MJ, Heinonen M (2002) J Agric Food Chem 50:7461–7466
Nuñez V, Monagas M, Gómez-Cordovés MC, Bartolomé B (2004) Postharvest Biol Technol 31:69–79
Martínez JA, Melgosa M, Pérez M, Hita E, Negueruela AI (2001) Food Sci Technol Int 7 5:439–444
Acknowledgments
Financial support received from INIA (Grant ref. VIN03-043-C3) and CICYT (Grant ref. AGL2002-00167) is greatly acknowledged. Author M. Dueñas thanks the Spanish Juan de la Cierva program for a grant and Mr. G. H. Jenkins for his help with the English version of the ms.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Manzano, S., Santos-Buelga, C., Dueñas, M. et al. Colour implications of self-association processes of wine anthocyanins. Eur Food Res Technol 226, 483–490 (2008). https://doi.org/10.1007/s00217-007-0560-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0560-9