Abstract
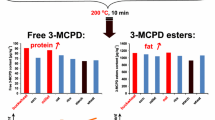
Formation of 3-chloropropane-1,2-diol (3-MCPD) from the precursors glycerol, triolein and soy lecithin in the presence of sodium chloride was studied. The precursors were reacted with sodium chloride in an emulsion stabilised with an emulsifier under conditions which modelled the thermal treatment of foods during processing. Three sets of experiments were carried out aimed to monitor the influence of various factors (NaCl, water content and temperature) on the yield of 3-MCPD. The formation of 3-MCPD strongly depended on the concentration of NaCl and reached a maximum level at about 4–7% NaCl. The highest amount of 3-MCPD was formed in media containing approximately 13–17% water. The amount of 3-MCPD increased with increasing temperature over the range 100–230 °C and reached its highest value at 230 °C. The production of 3-MCPD was also followed in models very closely related to selected foods which had been shown to have a high potential to yield 3-MCPD during processing (salami, beefburgers, processed cheese, biscuits, crackers, doughnuts). The highest levels of 3-MCPD were formed in models simulating salami as they had the highest content of both fat and salt of all the samples. The lowest amount of 3-MCPD was formed in the models simulating biscuits and crackers as they had a low salt content and, at the same time, their water content was below the optimum level.









Similar content being viewed by others
References
Velíšek J, Davídek J, Hajšlová J, Kubelka V, Janíček G, Mánková B (1978) Chlorohydrins in protein hydrolysates. Z Lebensm Unters Forsch 167:241–244
Velíšek J, Davídek J, Kubelka V, Bartošová J, Tučková A, Hajšlová J, Janíček G (1979) Formation of volatile chlorohydrins from glycerol (triacetin, tributyrin) and hydrochloric acid. Lebensm-Wiss u-Technol 12:234–236
Davídek J, Velíšek J, Kubelka V, Janíček G, Šimicová Z (1980) Glycerol chlorohydrins and their esters as products of the hydrolysis of tripalmitin, tristearin and triolein with hydrochloric acid. Z Lebensm Unters Forsch 171:14–17
Davídek J, Velíšek J, Kubelka V, Janíček G. (1982) New chlorine containing organic compounds in protein hydrolysates. Proc Euro Food Chem I, Vienna, Austria, 17–20 February, 1981. In: Baltes W, Czedik-Eysenberg PB, Pfannhauser W (eds) Recent developments in food analysis. Weinheim, Deerfield Beach, Florida, pp 322–325
Collier PD, Cromie DDO, Davies AP (1991) Mechanism of formation of chloropropanols present in protein hydrolysates. J Am Oil Chem Soc 68:785–790
Velíšek J, Doležal M, Crews C, Dvořák T (2002) Optical isomers of chloropropanols: mechanisms of their formation and decomposition in protein hydrolysates. Czech J Food Sci 20:161–170
Regulation 466/2001 coming into effect from 5th April 2002
Velíšek J, Davídek J, Šimicová Z, Svobodová Z (1982) Glycerol chlorohydrins and their esters-reaction products of lipids with hydrochloric acid. Sci Papers Prague Inst Chem Technol E53:55–65
Hamlet CG, Jayaratne SM, Matthews W (2002) 3-Monochloropropane-1,2-diol (3-MCPD) in food ingredients from UK food producers and ingredient suppliers. Food Addit Contamin 19:15–21
Crews C, Hough P, Brereton P, Harvey D, Macarthur R, Matthews W (2002) Survey of 3-monochloropeopane-1,2-diol (3-MCPD) in selected food groups, 1999–2000. Food Addit Contamin 19:22–27
Crews C, Brereton P, Davies A (2001) Effect of domestic cooking on the levels of 3-monochloropropane-1,2-diol in food. Food Addit Contamin 18:271–280
Warner K (1998) Chemistry of frying fats. In: Akoh CC, Min DB (eds) Food lipids. Marcel Dekker, New York Basel, pp 167–180
March J (1992) Advanced organic chemistry. Reactions, mechanisms and structure, 4th edn. Wiley, New York
Réblová Z (1998) Degradation of lipids at high temperatures (Degradace lipidů za vysoké teploty). PhD Thesis, ICT, Prague
Marmer NW (1985) Traditional and novel approaches to the analysis of plant phospholipids. In: Szuhaj FB, List RG (eds) Lecithins. American Oil Chemist’s Society, USA
Plantinga WJ, van Toorn WG, van der Stegen GHD (1991) Determination of 3-chloropropane-1,2-diol in liquid hydrolysed vegetable proteins by capillary gas chromatography with flame ionization detector. J Chromatogr 555:311–314
Acknowledgements
This work was supported partly by the UK Food Standards Agency, and partly by the research project MSM 223300004 granted by the Ministry of Education and Youth of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calta, P., Velíšek, J., Doležal, M. et al. Formation of 3-chloropropane-1,2-diol in systems simulating processed foods. Eur Food Res Technol 218, 501–506 (2004). https://doi.org/10.1007/s00217-003-0865-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-003-0865-2