Abstract
Acrylamide determination is important to state its quantity in baked food preventing any potential carcinogenic effects. Matrix solid-phase dispersion (MSPD) extraction is an extraction procedure based on a homogenization phase between a solid sample and a solid dispersing material to break sample increasing analyte extraction yield, often used for acrylamide determination. The addition of a green deep eutectic solvent (DES) during the MSPD homogenization phase improves the analyte extraction, giving the possibility to reduce the amount of organic solvent used. In this work, a miniaturized MSPD extraction assisted by a DES was developed to determine acrylamide in bread, using high-performance liquid chromatography coupled with mass spectrometry detection. The optimized procedure provides 1:1 (w/w) matrix-to-dispersing material ratio, 2 mL of methanol as extraction solvent, and 50 μL of choline chloride-glycerol DES added during the homogenization phase. Method validation ensured good results with minimum recoveries of 90%, high precision with a maximum intra-day error of 4%, and inter-day error of 6%. Limit of detection and limit of quantification resulted to be 16 μg/kg and 35 μg/kg, respectively. This miniaturized extraction procedure represents a good alternative to those reported in the literature, guaranteeing great performance and respecting green chemistry principles.
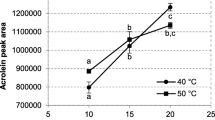
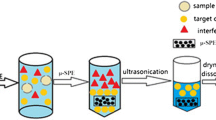
Graphical Abstract






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Zhou X, Duan M, Gao S, Wang T, Wang Y, Wang X e Zhou Y. A strategy for reducing acrylamide content in wheat bread by combining acidification rate and prerequisite substance content of Lactobacillus and Saccharomyces cerevisiae. Current Research in Food Science. 2022;5:1054–60. https://doi.org/10.1016/j.crfs.2022.06.005.
Sarion C, Codină GG, Dabija A. Acrylamide in bakery products: a review on health risks, legal regulations and strategies to reduce its formation. Int J Environ Res Public Health. 2021;18(8):4332. https://doi.org/10.3390/ijerph18084332.
Miśkiewicz K, Nebesny E, Rosicka-Kaczmarek J, Zyzelewicz D, Budryn G. The effects of baking conditions on acrylamide content in shortcrust cookies with added freeze-dried aqueous rosemary extract. J Food Sci Technol. 2018;55:4184–96. https://doi.org/10.1007/s13197-018-3349-x.
Cantrell MS, McDougal OM. Biomedical rationale for acrylamide regulation andmethods of detection. Compr Rev Food Sci. 2021;20:2176–205. https://doi.org/10.1111/1541-4337.12696.
Koszucka A, Nowak A, Nowak I, Motyl I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit Rev Food Sci Nutr. 2020;60(10):1677–92. https://doi.org/10.1080/10408398.2019.1588222.
Mollakhalili-Meybodi N, Khorshidian N, Nematollahi A, Arab M. Acrylamide in bread: a review on formation, health risk assessment, and determination by analytical techniques. Environ Sci Pollut Res. 2021;28:1562745. https://doi.org/10.1007/s11356-021-12775-3.
Mesias M, Delgado-Andrade C, Morales FJ. An updated view of acrylamide in cereal product. Curr Opin Food Sci. 2022;46: 100847. https://doi.org/10.1016/j.cofs.2022.100847.
Kumari A, Bhattacharya B, Agarwal T, Paul V, Maurya VK, Chakkaravarthi S, Simal-Gandara J. Method development and validation for acrylamide in potato cutlet by UHPLC-MS/MS. Food Control. 2023;151: 109817. https://doi.org/10.1016/j.foodcont.2023.109817.
Lindsay RC, Jang S. Model systems for evaluating factors affecting acrylamide formation in deep fried foods. In: Friedman, M., Mottram, D. (eds). Chemistry and safety of acrylamide in food. Advances in Experimental Medicine and Biology. Boston: Springer 561. https://doi.org/10.1007/0-387-24980-X_25.
Wang S, Yu J, Xin Q, Wang S, Copeland L. Effects of starch damage and yeast fermentation on acrylamide formation in bread. Food Control. 2017;73(B):230–6. https://doi.org/10.1016/j.foodcont.2016.08.002.
Saraji M, Javadian S. Single-drop microextraction combined with gas chromatography-electron capture detection for the determination of acrylamide in food samples. Food Chem. 2019;274:55–60. https://doi.org/10.1016/j.foodchem.2018.08.108.
Norouzi E, Kamankesh M, Mohammadi A, Attaran A. Acrylamide in bread samples: determining using ultrasonic-assisted extraction and microextraction method followed by gas chromatography-mass spectrometry. J Cereal Sci. 2018;79:1–5. https://doi.org/10.1016/j.jcs.2017.09.011.
Hoff RB, Pizzolato TM. Combining extraction and purification steps in sample preparation for environmental matrices: a review of matrix solid phase dispersion (MSPD) and pressurized liquid extraction (PLE) applications. Trends Analyt Chem. 2018;109:83–96. https://doi.org/10.1016/j.trac.2018.10.002.
Oracz J, Nebesny E, Żyżelewicz D. New trends in quantification of acrylamide in food products. Talanta. 2011;86:23–34. https://doi.org/10.1016/j.talanta.2011.08.066.
Xu XM, He HL, Zhu Y, Feng L, Ying Y, Huang BF, Shen HT, Han JL, Ren YP. Simultaneous determination of 3-monochloropropane-1,2-diol and acrylamide in food by gas chromatography-triple quadrupole mass spectrometry with coupled column separation. Anal Chim. 2013;760:93–9. https://doi.org/10.1016/j.aca.2012.11.036.
Wianowska D, Gil M. New insights into the application of MSPD in various fields of analytical chemistry. Trends Analyt Chem. 2021;2021(112):29–51. https://doi.org/10.1016/j.trac.2018.12.028.
Arabi M, Ghaedi M, Ostovan A. Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chem. 2016;210:78–84. https://doi.org/10.1016/j.foodchem.2016.04.080.
Soares CMD, Fernandes JO. MSPD method to determine acrylamide in food. J Anal Methods Chem. 2009;2:197–203. https://doi.org/10.1007/s12161-008-9060-1.
Zhao H, Li N, Li J, Qiao X e Xu Z. Preparation and application of chitosan-grafted multiwalled carbon nanotubes in matrix solid-phase dispersion extraction for determination of trace acrylamide in foods through high-performance liquid chromatography. Food Anal. Methods. 2015; 8:1363–71. https://doi.org/10.1007/s12161-014-0022-5.
Danek M, Fang X, Tang J, Plonka J, Barchanska H. Simultaneous determination of pesticides and their degradation products in potatoes by MSPD-LC-MS/MS. J Food Compos Anal. 2021;104: 104129. https://doi.org/10.1016/j.jfca.2021.104129.
Capriotti AL, Cavaliere C, Foglia P, Samperi R, Stampachiacchiere S, Ventura S, Laganà A. Recent advances and developments in matrix solid-phase dispersion. Trends Analyt Chem. 2015;71:186–93. https://doi.org/10.1016/j.trac.2015.03.012.
El Achkar T, Greige-Gerges H, Fourmentin S. Basics and properties of deep eutectic solvents: a review. Environ Chem Lett. 2021;19:3397–408. https://doi.org/10.1007/s10311-021-01225-8.
Haq HU, Bibi R, Arain MB, SafiF, Ullah S, Castro-Muñoz R, Boczkaj G. Deep eutectic solvent (DES) with silver nanoparticles (Ag-NPs) based assay for analysis of lead (II) in edible oils. Food Chem. 2022;379:132085. https://doi.org/10.1016/j.foodchem.2022.132085.
Li X, Row KH. Development of deep eutectic solvents applied in extraction and separation. J Sep Sci. 2016;39(18):3505–20. https://doi.org/10.1002/jssc.201600633.
Janjhi FA, Castro-Muñoz R, Boczkaj G. Deep eutectic solvents – ideal solution for clean air or hidden danger? Sep Purif Technol. 2023;314: 123590. https://doi.org/10.1016/j.seppur.2023.123590.
Marchel M, Cieśliński H, Boczkaj G. Deep eutectic solvents microbial toxicity: current state of art and critical evaluation of testing methods. J Hazard Mater. 2022;425: 127963. https://doi.org/10.1016/j.jhazmat.2021.127963.
Ivanovic M, Razboršek MI, Kolar M. Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants. 2020;9 (11)1428. https://doi.org/10.3390/plants9111428.
Fanali C, Della Posta S, Dugo L, Gentili A, Mondello L, De Gara L. Choline-chloride and betaine-based deep eutectic solvents for green extraction of nutraceutical compounds from spent coffee ground. J Pharm Biomed Anal. 2020;189: 113421. https://doi.org/10.1016/j.jpba.2020.113421.
Hu C, Feng J, Cao Y, Chen L, Li Y. Deep eutectic solvents in sample preparation and determination methods of pesticides: recent advances and future prospects. Talanta. 2024;266(Pt 2): 125092. https://doi.org/10.1016/j.talanta.2023.125092.
Osiecka D, Vakh C, Makoś-Chełstowska P, Kubica P. Plant-based meat substitute analysis using microextraction with deep eutectic solvent followed by LC-MS/MS to determine acrylamide, 5-hydroxymethylfurfural and furaneol. Anal Bioanal Chem. 2024;416:1117–26. https://doi.org/10.1007/s00216-023-05107-6.
Nedaei M, Zarei AR, Ghorbanian SA. Miniaturized matrix solid-phase dispersion based on deep eutectic solvent and carbon nitride associated with high-performance liquid chromatography: a new feasibility for extraction and determination of trace nitrotoluene pollutants in soil samples. J Chromatogr A. 2019;1601:35–44. https://doi.org/10.1016/j.chroma.2019.05.008.
Wu X, Zhang X, Yang Y, Liu Y, Chen X. Development of a deep eutectic solvent-based matrix solid phase dispersion methodology for the determination of aflatoxins in crops. Food Chem. 2019;291:239–44. https://doi.org/10.1016/j.foodchem.2019.04.030.
Yang F, Jiang L, Mao H, Zou Y, Chu C. Establishment of deep eutectic solvent-assisted matrix solid-phase dispersion extraction for the determination of four flavonoids in Scutellariae Radix based on the concept of quality by design. J AOAC Int. 2021;104:1681–9. https://doi.org/10.1093/jaoacint/qsab043.
Funding
This study was funded by PRO-FORNO project (n. PROT. A0375-2020–36570) of Regione Lazio, Italy, Avviso Pubblico “Gruppi di ricerca 2020”—POR FESR Lazio 2014–2020—Azione 1.2.
Author information
Authors and Affiliations
Contributions
Susanna Della Posta (data collection, analysis, writing—original draft preparation), Anna Maria Ascrizzi (material preparation, data collection, analysis, draft), Giorgia Pietrangeli (analysis, draft), Vittoria Terrigno (draft), Elisa De Arcangelis (review and editing), Stefania Ruggeri (review and editing), Chiara Fanali (supervision, conceptualization, writing—original draft preparation).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Sustainability in Sample Preparation with guest editors Soledad Cárdenas and Pablo Richter.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Della Posta, S., Ascrizzi, A.M., Pietrangeli, G. et al. Miniaturized matrix solid-phase dispersion assisted by deep eutectic solvent for acrylamide determination in bread samples. Anal Bioanal Chem (2024). https://doi.org/10.1007/s00216-024-05315-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00216-024-05315-8