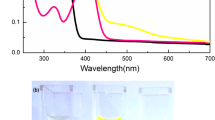
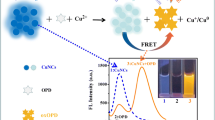
Abstract
When Cu(II) reacts with ascorbic acid (AA) to form Cu(I), Cu(I) can combine with eosin Y (EY) to form ionic associations, resulting in significant fluorescence quenching of the EY. Based on the turn-off of fluorescence in the chemosensor EY, a green reaction is proposed herein for the detection of Cu(II). The novel detection method for Cu(II) demonstrates simplicity, high sensitivity, and excellent selectivity, rendering it suitable for analyzing environmental samples. A static fluorescence quenching mechanism is validated through the Stern–Volmer relationship, and the thermodynamic parameters of the reaction are explored using a van 't Hoff plot. The reaction mechanism is investigated via fluorescence spectra, absorption spectra, and density-functional theory (DFT) calculations. The probe's green nature is confirmed by applying four green analytical chemistry metrics.









Similar content being viewed by others
References
Barceloux DG. Copper. J Toxicol Clin Toxicol. 1999;37(2):217–30. https://doi.org/10.1081/clt-100102421.
Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev. 2006;106(6):1995–2044. https://doi.org/10.1021/cr040410w.
Harrison MD, Dameron CT. Molecular mechanisms of copper metabolism and the role of the Menkes disease protein. J Biochem Mol Toxicol. 1999;13(2):93–106. https://doi.org/10.1002/(sici)1099-0461(1999)13:2%3c93::aid-jbt5%3e3.0.co;2-3.
Strausak D, Mercer JF, Dieter HH, Stremmel W, Multhaup G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull. 2001;55(2):175–85. https://doi.org/10.1016/s0361-9230(01)00454-3.
Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol Dis. 1999;6(4):221–30. https://doi.org/10.1006/nbdi.1999.0250.
Tamura T, Turnlund JR. Effect of long-term, high-copper intake on the concentrations of plasma homocysteine and B vitamins in young men. Nutrition. 2004;20(9):757–9. https://doi.org/10.1016/j.nut.2004.05.011.
Wu SM, Jong KJ, Kuo SY. Effects of copper sulfate on ion balance and growth in tilapia larvae (Oreochromis mossambicus). Arch Environ Contam Toxicol. 2003;45(3):357–63. https://doi.org/10.1007/s00244-003-0122-5.
Tavares-Dias M. Toxic, physiological, histomorphological, growth performance and antiparasitic effects of copper sulphate in fish aquaculture. Aquaculture. 2021;535:736350. https://doi.org/10.1016/j.aquaculture.2021.736350.
Organization GWH (2017) Guidelines for drinking-water quality: fourth edition incorporating the first addendum. License: CC BY-NC-SA 30 IGO nd.
Kim DB, Hong JM, Chang S-K. Colorimetric determination of Cu2+ ions with a desktop scanner using silica nanoparticles via formation of a quinonediimine dye. Sensor Actuat B-Chem. 2017;252:537–43. https://doi.org/10.1016/j.snb.2017.06.033.
Xu L, Wei S, Diao Q, Ma P, Liu X, Sun Y, Song D, Wang X. Sensitive and selective rhodamine-derived probes for fluorometric sensing of pH and colorimetric sensing of Cu2+. Sensor Actuat B-Chem. 2017;246:395–401. https://doi.org/10.1016/j.snb.2017.02.093.
Kaur M, Cho MJ, Choi DH. A phenothiazine-based “naked-eye” fluorescent probe for the dual detection of Hg2+ and Cu2+: Application as a solid state sensor. Dyes Pigments. 2016;125:1–7. https://doi.org/10.1016/j.dyepig.2015.09.030.
Xie D-H, Wang X-J, Sun C, Han J. Calix[4]arene based 1,3,4-oxadiazole as a fluorescent chemosensor for copper(II) ion detection. Tetrahedron Lett. 2016;57(51):5834–6. https://doi.org/10.1016/j.tetlet.2016.11.051.
Qiu X, Han S, Hu Y, Gao M, Wang H. Periodic mesoporous organosilicas for ultra-high selective copper(ii) detection and sensing mechanism. J Mater Chem A. 2014;2(5):1493–501. https://doi.org/10.1039/c3ta14314g.
Dai X, Wu QH, Wang PC, Tian J, Xu Y, Wang SQ, Miao JY, Zhao BX. A simple and effective coumarin-based fluorescent probe for cysteine. Biosens Bioelectron. 2014;59:35–9. https://doi.org/10.1016/j.bios.2014.03.018.
Soylak M, Narin I, Dogan M. Trace enrichment and atomic absorption spectrometric determination of lead, copper, cadmium and nickel in drinking water samples by use of an activated carbon column. Anal Lett. 2006;30(15):2801–10. https://doi.org/10.1080/00032719708001823.
Ghaedi M, Ahmadi F, Shokrollahi A. Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J Hazard Mater. 2007;142(1–2):272–8. https://doi.org/10.1016/j.jhazmat.2006.08.012.
Liu Y, Liang P, Guo L. Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta. 2005;68(1):25–30. https://doi.org/10.1016/j.talanta.2005.04.035.
Bings NH, Bogaerts A, Broekaert JA. Atomic spectroscopy. Anal Chem. 2006;78(12):3917–46. https://doi.org/10.1021/ac060597m.
Ensafi AA, Khayamian T, Benvidi A, Mirmomtaz E. Simultaneous determination of copper, lead and cadmium by cathodic adsorptive stripping voltammetry using artificial neural network. Anal Chim Acta. 2006;561(1–2):225–32. https://doi.org/10.1016/j.aca.2006.01.015.
Shi DT, Zhang B, Yang YX, Guan CC, He XP, Li YC, Chen GR, Chen K. Bis-triazolyl indoleamines as unique “off-approach-on” chemosensors for copper and fluorine. Analyst. 2013;138(10):2808–11. https://doi.org/10.1039/c3an00030c.
Quan L, Sun T, Lin W, Guan X, Zheng M, Xie Z, Jing X. BODIPY fluorescent chemosensor for Cu2+ detection and its applications in living cells: fast response and high sensitivity. J Fluoresc. 2014;24(3):841–6. https://doi.org/10.1007/s10895-014-1360-9.
Paun A, Hadade ND, Paraschivescu CC, Matache M. 1,3,4-Oxadiazoles as luminescent materials for organic light emitting diodes via cross-coupling reactions. J Mater Chem C. 2016;4(37):8596–610. https://doi.org/10.1039/c6tc03003c.
Erdemir S, Tabakci B, Tabakci M. A highly selective fluorescent sensor based on calix[4]arene appended benzothiazole units for Cu2+, S2− and HSO4− ions in aqueous solution. Sensor Actuat B-Chem. 2016;228:109–16. https://doi.org/10.1016/j.snb.2016.01.017.
Jin LH, Han CS. Ultrasensitive and selective fluorimetric detection of copper ions using thiosulfate-involved quantum dots. Anal Chem. 2014;86(15):7209–13. https://doi.org/10.1021/ac501515f.
Nozik AJ, Beard MC, Luther JM, Law M, Ellingson RJ, Johnson JC. Semiconductor quantum dots and quantum dot arrays and applications of multiple exciton generation to third-generation photovoltaic solar cells. Chem Rev. 2010;110(11):6873–90. https://doi.org/10.1021/cr900289f.
Wang Y, Hu A. Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2014;2 (34). https://doi.org/10.1039/c4tc00988f
Cui J, Wang S, Huang K, Li Y, Zhao W, Shi J, Gu J. Conjugation-induced fluorescence labelling of mesoporous silica nanoparticles for the sensitive and selective detection of copper ions in aqueous solution. New J Chem. 2014;38(12):6017–24. https://doi.org/10.1039/c4nj01428f.
Li Y, Dong C, Chu J, Qi J, Li X. Surface molecular imprinting onto fluorescein-coated magnetic nanoparticles via reversible addition fragmentation chain transfer polymerization: a facile three-in-one system for recognition and separation of endocrine disrupting chemicals. Nanoscale. 2011;3(1):280–7. https://doi.org/10.1039/c0nr00614a.
Lee I, Kim S, Kim SN, Jang Y, Jang J. Highly fluorescent amidine/schiff base dual-modified polyacrylonitrile nanoparticles for selective and sensitive detection of copper ions in living cells. ACS Appl Mater Interfaces. 2014;6(19):17151–6. https://doi.org/10.1021/am504824n.
Van der Voort P, Esquivel D, De Canck E, Goethals F, Van Driessche I, Romero-Salguero FJ. Periodic Mesoporous Organosilicas: from simple to complex bridges; a comprehensive overview of functions, morphologies and applications. Chem Soc Rev. 2013;42(9):3913–55. https://doi.org/10.1039/c2cs35222b.
Mahapatra AK, Mondal S, Manna SK, Maiti K, Maji R, Uddin MR, Mandal S, Sarkar D, Mondal TK, Maiti DK. A new selective chromogenic and turn-on fluorogenic probe for copper(II) in solution and vero cells: recognition of sulphide by [CuL]. Dalton Trans. 2015;44(14):6490–501. https://doi.org/10.1039/c4dt03969f.
Mei Y, Bentley PA, Wang W. A selective and sensitive chemosensor for Cu2+ based on 8-hydroxyquinoline. Tetrahedron Lett. 2006;47(14):2447–9. https://doi.org/10.1016/j.tetlet.2006.01.091.
Chou CY, Liu SR, Wu SP. A highly selective turn-on fluorescent sensor for Cu(II) based on an NSe2 chelating moiety and its application in living cell imaging. Analyst. 2013;138(11):3264–70. https://doi.org/10.1039/c3an00286a.
Mayo SL, Olafson BD, Goddard WA. DREIDING: a generic force field for molecular simulations. J Phys Chem C. 1990;94(26):8897–909.
Sokalski WA, Poirier R. Cumulative atomic multipole representation of the molecular charge distribution and its basis set dependence. Chem Phys Lett. 1983;98(1):86–92.
Autschbach J, Ziegler T, van Gisbergen SJ, Baerends EJ. Chiroptical properties from time-dependent density functional theory. I. Circular dichroism spectra of organic molecules. J Chem Phys. 2002;116(16):6930–40.
Helgaker T, Jo P. An electronic Hamiltonian for origin independent calculations of magnetic properties. J Chem Phys. 1991;95(4):2595–601.
Bak KL, Jo P, Helgaker T, Ruud K, Jo H. Gauge-origin independent multiconfigurational self-consistent-field theory for vibrational circular dichroism. J Chem Phys. 1993;98(11):8873–87.
Cossi M, Scalmani G, Rega N, Barone V. New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J Chem Phys. 2002;117(1):43–54.
Barone V, Cossi M, Tomasi J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J Chem Phys. 1997;107(8):3210–21.
Batistela VR, Pellosi DS, de Souza FD, da Costa WF, de Oliveira Santin SM, de Souza VR, Caetano W, de Oliveira HP, Scarminio IS, Hioka N. pKa determinations of xanthene derivates in aqueous solutions by multivariate analysis applied to UV-Vis spectrophotometric data. Spectrochim Acta A. 2011;79(5):889–97. https://doi.org/10.1016/j.saa.2011.03.027.
Zhang F, Shi F, Ma W, Gao F, Jiao Y, Li H, Wang J, Shan X, Lu X, Meng S. Controlling adsorption structure of eosin Y dye on nanocrystalline TiO2 films for improved photovoltaic performances. J Phys Chem C. 2013;117(28):14659–66. https://doi.org/10.1021/jp404439p.
Wang Y-Q, Zhang H-M, Zhang G-C, Tao W-H, Tang S-H. Interaction of the flavonoid hesperidin with bovine serum albumin: a fluorescence quenching study. J Lumin. 2007;126(1):211–8. https://doi.org/10.1016/j.jlumin.2006.06.013.
Lakowicz JR. Principles of Fluorescence Spectroscopy. Third edition edn. Springer, NY 2006.
Komalam A, Muraleegharan LG, Subburaj S, Suseela S, Babu A, George S. Designed plasmonic nanocatalysts for the reduction of eosin Y: absorption and fluorescence study. Int Nano Lett. 2012;2(1):26. https://doi.org/10.1186/2228-5326-2-26.
Fita P, Fedoseeva M, Vauthey E. Ultrafast excited-state dynamics of eosin B: a potential probe of the hydrogen-bonding properties of the environment. J Phys Chem A. 2011;115(12):2465–70. https://doi.org/10.1021/jp110849x.
R. Chang JWTJ. Physical Chemistry for the Chemical Science, University Science Books, Canada 2014.
Wang J, Chen L, Li Y, Shen W, Manley-Harris M. A novel determination method for Ag(I) in environmental samples based on reduction of absorbance and fluorescence quenching of Eosin Y. Microchem J. 2024;196:109588. https://doi.org/10.1016/j.microc.2023.109588.
Kaur N, Kaur G, Alreja P. 1, 10-Phenanthroline based ESIPT sensor for cascade recognition of Cu2+ and CN− ions. J Photoch Photobio A. 2018;353:138–42. https://doi.org/10.1016/j.jphotochem.2017.11.012.
Said AI, Georgiev NI, Bojinov VB. The simplest molecular chemosensor for detecting higher pHs, Cu2+ and S2- in aqueous environment and executing various logic gates. J Photoch Photobio A. 2019;371:395–406. https://doi.org/10.1016/j.jphotochem.2018.11.029.
Kaur B, Gupta A, Kaur N. A simple schiff base as a multi responsive and sequential sensor towards Al3+, F− and Cu2+ ions. J Photoch Photobio A. 2020;389:112140. https://doi.org/10.1016/j.jphotochem.2019.112140.
Wang JX, Xing ZY, Tian ZN, Wu DQ, Xiang YY, Li JL. A dual-functional probe for sensing pH change and ratiometric detection of Cu(2). Spectrochim Acta A. 2020;235:118318. https://doi.org/10.1016/j.saa.2020.118318.
Zhang B, Diao Q, Ma P, Liu X, Song D, Wang X. A sensitive fluorescent probe for Cu2+ based on rhodamine B derivatives and its application to drinking water examination and living cells imaging. Sensor Actuat B-Chem. 2016;225:579–85. https://doi.org/10.1016/j.snb.2015.11.069.
Wang M, Zhang D, Li M, Fan M, Ye Y, Zhao YF. A rhodamine-cyclen conjugate as chromogenic and fluorescent chemosensor for copper ion in aqueous media. J Fluoresc. 2013;23(3):417–23. https://doi.org/10.1007/s10895-013-1159-0.
Wang W, Wang X, Yang Q, Fei X, Sun M, Song Y. A reusable nanofibrous film chemosensor for highly selective and sensitive optical signaling of Cu2+ in aqueous media. Chem Commun (Camb). 2013;49(42):4833–5. https://doi.org/10.1039/c3cc41317a.
Chen Y, Zhu C, Cen J, Li J, He W, Jiao Y, Guo Z. A reversible ratiometric sensor for intracellular Cu2+ imaging: metal coordination-altered FRET in a dual fluorophore hybrid. Chem Commun (Camb). 2013;49(69):7632–4. https://doi.org/10.1039/c3cc42959h.
Fan J, Zhan P, Hu M, Sun W, Tang J, Wang J, Sun S, Song F, Peng X. A fluorescent ratiometric chemodosimeter for Cu2+ based on TBET and its application in living cells. Org Lett. 2013;15(3):492–5. https://doi.org/10.1021/ol3032889.
Tuzen M, Soylak M, Citak D, Ferreira HS, Korn MG, Bezerra MA. A preconcentration system for determination of copper and nickel in water and food samples employing flame atomic absorption spectrometry. J Hazard Mater. 2009;162(2–3):1041–5. https://doi.org/10.1016/j.jhazmat.2008.05.154.
Salinas-Castillo A, Ariza-Avidad M, Pritz C, Camprubi-Robles M, Fernandez B, Ruedas-Rama MJ, Megia-Fernandez A, Lapresta-Fernandez A, Santoyo-Gonzalez F, Schrott-Fischer A, Capitan-Vallvey LF. Carbon dots for copper detection with down and upconversion fluorescent properties as excitation sources. Chem Commun (Camb). 2013;49(11):1103–5. https://doi.org/10.1039/c2cc36450f.
Kalanidhi K, Nagaraaj P, Aswathy CA, VanthanaSree G. A highly selective and sensitive spectroscopic method for detection of Cu2+ in aqueous solution using polyaniline. Chem Phys Lett. 2020;739:136929. https://doi.org/10.1016/j.cplett.2019.136929.
Abbasse G, Ouddane B, Fischer JC. Determination of total and labile fraction of metals in seawater using solid phase extraction and inductively coupled plasma atomic emission spectrometry (ICP-AES). J Anal At Spectrom. 2002;17(10):1354–8. https://doi.org/10.1039/b203407g.
Keith LH, Gron LU, Young JL. Green analytical methodologies. Chem Rev. 2007;107(6):2695–708. https://doi.org/10.1021/cr068359e.
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC-Trends in Anal Chem. 2012;37:61–72. https://doi.org/10.1016/j.trac.2012.03.013.
Plotka-Wasylka J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta. 2018;181:204–9. https://doi.org/10.1016/j.talanta.2018.01.013.
Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE-analytical GREEnness metric approach and software. Anal Chem. 2020;92(14):10076–82. https://doi.org/10.1021/acs.analchem.0c01887.
Author information
Authors and Affiliations
Contributions
Jian Wang: Conceptualization, Methodology, Investigation, Writing the original draft, Visualization; Ling Chen: Investigation, Visualization; Yanan Li: Investigation, Writing the original draft; Merilyn Manley-Harris: Methodology, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Chen, L., Li, Y. et al. A green reaction-based turn-off fluorescence sensor for determination of copper ions: DFT calculations, quenching mechanism, green chemistry metrics, and application in environmental samples. Anal Bioanal Chem (2024). https://doi.org/10.1007/s00216-024-05293-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00216-024-05293-x