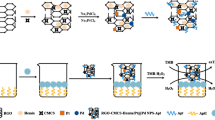
Abstract
α-Glucosidase (α-Glu) is implicated in the progression and pathogenesis of type II diabetes (T2D). In this study, we developed a rapid colorimetric technique using platinum nanoparticles stabilized by chitosan (Ch-PtNPs) to detect α-Glu activity and its inhibitor. The Ch-PtNPs facilitate the conversion of 3,3′,5,5′-tetramethylbenzidine (TMB) into oxidized TMB (oxTMB) in the presence of dissolved O2. The catalytic hydrolysis of 2-O-α-d-glucopyranosyl-l-ascorbic acid (AA-2G) by α-Glu produces ascorbic acid (AA), which reduces oxTMB to TMB, leading to the fading of the blue color. However, the presence of α-Glu inhibitors (AGIs) hinders the generation of AA, allowing Ch-PtNPs to re-oxidize colorless TMB back to blue oxTMB. This unique phenomenon enables the colorimetric detection of α-Glu activity and AGIs. The linear range for α-Glu was found to be 0.1–1.0 U mL−1 and the detection limit was 0.026 U mL−1. Additionally, the half-maximal inhibition value (IC50) for acarbose, an α-Glu inhibitor, was calculated to be 0.4769 mM. Excitingly, this sensing platform successfully detected α-Glu activity in human serum samples and effectively screened AGIs. These promising findings highlight the potential application of the proposed strategy in clinical diabetes diagnosis and drug discovery.
Graphical Abstract






Similar content being viewed by others
References
Liu B, Ma JM, Chen HW, Li ZL, Sun LH, Zeng Z, Jiang H. The α-glucosidase inhibitory activities of phenolic acid amides with l-amino acid moiety. RSC Adv. 2016;6(56):50837–45. https://doi.org/10.1039/c6ra08330g.
Singh V, Krishnan S. Electrochemical and surface plasmon insulin assays on clinical samples. Analyst. 2018;143(7):1544–55. https://doi.org/10.1039/c7an01872j.
Swapna G, Soman KP, Vinayakumar R. Automated detection of diabetes using CNN and CNN-LSTM network and heart rate signals. Procedia Computer Science. 2018;132:1253–62. https://doi.org/10.1016/j.procs.2018.05.041.
Ruan CT, Lam SH, Lee SS, Su MJ. Hypoglycemic action of borapetoside A from the plant Tinospora crispa in mice. Phytomedicine. 2013;20(8–9):667–75. https://doi.org/10.1016/j.phymed.2013.02.009.
Liu DM, Dong C, Ma RT. A colorimetric method for screening alpha-glucosidase inhibitors from flavonoids using 3,3′,5,5′-tetramethylbenzidine as a chromogenic probe. Colloid Surface B. 2021;197:111400. https://doi.org/10.1016/j.colsurfb.2020.111400.
Li YS, Li XT, Yu LG, Wang L, Shi ZY, Guo XL. Roles of galectin-3 in metabolic disorders and tumor cell metabolism. Int J Biol Macromol. 2020;142:463–73. https://doi.org/10.1016/j.ijbiomac.2019.09.118.
Mahmoud AM, Geslevich J, Kint J, Depuydt C, Huysse L, Zalata A, Comhaire FH. Seminal plasma alpha-glucosidase activity and male infertility. Hum Reprod. 1998;13(3):591–5. https://doi.org/10.1093/humrep/13.3.591.
Simoes-Pires CA, Hmicha B, Marston A, Hostettmann K. A TLC bioautographic method for the detection of alpha- and beta-glucosidase inhibitors in plant extracts. Phytochem Anal. 2009;20(6):511–5. https://doi.org/10.1002/pca.1154.
Cheng X, Huang Y, Yuan C, Dai K, Jiang H, Ma JM. Colorimetric detection of α-glucosidase activity based on the etching of gold nanorods and its application to screen anti-diabetic drugs. Sensor Actuat B-Chem. 2019;282:838–43. https://doi.org/10.1016/j.snb.2018.11.097.
Zhang J, Liu Y, Lv J, Li GX. A colorimetric method for α-glucosidase activity assay and its inhibitor screening based on aggregation of gold nanoparticles induced by specific recognition between phenylenediboronic acid and 4-aminophenyl-α-d-glucopyranoside. Nano Res. 2014;8(3):920–30. https://doi.org/10.1007/s12274-014-0573-1.
Sun J, Yang F, Zhao D, Yang XR. Highly sensitive real-time assay of inorganic pyrophosphatase activity based on the fluorescent gold nanoclusters. Anal Chem. 2014;86(15):7883–9. https://doi.org/10.1021/ac501814u.
Li JL, He GW, Wang B, Shi L, Gao T, Li GX. Fabrication of reusable electrochemical biosensor and its application for the assay of alpha-glucosidase activity. Anal Chim Acta. 2018;1026:140–6. https://doi.org/10.1016/j.aca.2018.04.015.
Mohiuddin M, Arbain D, Islam AKMS, Ahmad MS, Ahmad MN. Alpha-glucosidase enzyme biosensor for the electrochemical measurement of antidiabetic potential of medicinal plants. Nanoscale Res Lett. 2016;11(1):95. https://doi.org/10.1186/s11671-016-1292-1.
Jiang JB, Li JJ, Zhu JL, Yu YJ, Duan GL, Zhou LG, Li Y. Synthesis of sandwich-structured magnetic graphene-Zn-MOFs composites for quantitative determination of acarbose in rat plasma. Talanta. 2020;209:120514. https://doi.org/10.1016/j.talanta.2019.120514.
Siebert DA, Campos JS, Alberton MD, Vitali L, Micke GA. Dual electrophoretically-mediated microanalysis in multiple injection mode for the simultaneous determination of acetylcholinesterase and alpha-glucosidase activity applied to selected polyphenols. Talanta. 2021;224:121773. https://doi.org/10.1016/j.talanta.2020.121773.
Ofosu FK, Elahi F, Daliri EB, Tyagi A, Chen XQ, Chelliah R, Kim JH, Han SI, Oh DH. UHPLC-ESI-QTOF-MS/MS characterization, antioxidant and antidiabetic properties of sorghum grains. Food Chem. 2021;337:127788. https://doi.org/10.1016/j.foodchem.2020.127788.
Ni MT, Hu X, Gong DM, Zhang GW. Inhibitory mechanism of vitexin on α-glucosidase and its synergy with acarbose. Food Hydrocolloid. 2020;105:824. https://doi.org/10.1016/j.foodhyd.2020.105824.
Li GL, Kong WH, Zhao M, Lu SM, Gong PW, Chen G, Xia L, Wang H, You JM, Wu YN. A fluorescence resonance energy transfer (FRET) based “Turn-On” nanofluorescence sensor using a nitrogen-doped carbon dot-hexagonal cobalt oxyhydroxide nanosheet architecture and application to alpha-glucosidase inhibitor screening. Biosens Bioelectron. 2016;79:728–35. https://doi.org/10.1016/j.bios.2015.12.094.
Shi ML, Cen Y, Xu GH, Wei FD, Xu XM, Cheng X, Chai YY, Sohail M, Hu Q. Ratiometric fluorescence monitoring of alpha-glucosidase activity based on oxidase-like property of MnO2 nanosheet and its application for inhibitor screening. Anal Chim Acta. 2019;1077:225–31. https://doi.org/10.1016/j.aca.2019.05.037.
Kong WH, Wu D, Xia L, Chen XF, Li GL, Qiu NN, Chen G, Sun ZW, You JM, Wu YN. Carbon dots for fluorescent detection of alpha-glucosidase activity using enzyme activated inner filter effect and its application to anti-diabetic drug discovery. Anal Chim Acta. 2017;973:91–9. https://doi.org/10.1016/j.aca.2017.03.050.
Wang MJ, Wang MK, Zhang F, Su XG. A ratiometric fluorescent biosensor for the sensitive determination of alpha-glucosidase activity and acarbose based on N-doped carbon dots. Analyst. 2020;145(17):5808–15. https://doi.org/10.1039/d0an01065k.
Zhang J, Liu Y, Wang XN, Chen YY, Li GX. Electrochemical assay of alpha-glucosidase activity and the inhibitor screening in cell medium. Biosens Bioelectron. 2015;74:666–72. https://doi.org/10.1016/j.bios.2015.07.023.
Xie JX, Zhang XD, Wang H, Zheng HZ, Huang YM. Analytical and environmental applications of nanoparticles as enzyme mimetics. TrAC-Trend Anal Chem. 2012;39:114–29. https://doi.org/10.1016/j.trac.2012.03.021.
Lin YH, Ren JS, Qu XG. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res. 2014;47(4):1097–105. https://doi.org/10.1021/ar400250z.
Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42(14):6060–93. https://doi.org/10.1039/c3cs35486e.
Wang Y, Li T, Wei H. Determination of the maximum velocity of a peroxidase-like nanozyme. Anal Chem. 2023;95(26):10105–9. https://doi.org/10.1021/acs.analchem.3c01830.
Wang Y, Yin L, Qu G, Leung CH, Han L, Lu L. Highly active single-atom nanozymes with high-loading iridium for sensitive detection of pesticides. Anal Chem. 2023;95(32):11960–8. https://doi.org/10.1021/acs.analchem.3c01569.
Xu H, Guo J, Zhao J, Gao Z, Song YY. Enantioselective target transport-mediated nanozyme decomposition for the identification of reducing enantiomers in asymmetric nanochannel arrays. Anal Chem. 2023;95(38):14465–74. https://doi.org/10.1021/acs.analchem.3c03089.
Li G, Li X, Xu W, Li S, Tan X, Liang J, Zhou Z. Reduced graphene oxide-persimmon tannin /Pt@Pd nanozyme-based cascade colorimetric sensor for detection of 1,5-anhydroglucitol. Anal Bioanal Chem. 2023;415(29–30):7103–15. https://doi.org/10.1007/s00216-023-04975-2.
Lyu Z, Zhou J, Ding S, Du D, Wang J, Liu Y, Lin Y. Recent advances in single-atom nanozymes for colorimetric biosensing. TrAC-Trend Anal Chem. 2023;168:117280. https://doi.org/10.1016/j.trac.2023.117280.
Ikeda S, Ishino S, Harada T, Okamoto N, Sakata T, Mori H, Kuwabata S, Torimoto T, Matsumura M. Ligand-free platinum nanoparticles encapsulated in a hollow porous carbon shell as a highly active heterogeneous hydrogenation catalyst. Angew Chem. 2006;118(42):7221–4. https://doi.org/10.1002/ange.200602700.
Mu YY, Liang HP, Hu JS, Jiang L, Wan LJ. Controllable Pt nanoparticle deposition on carbon nanotubes as an anode catalyst for direct methanol fuel cells. J Phys Chem B. 2005;109(47):22212–6. https://doi.org/10.1021/jp0555448.
Lee JY, Liao HW, Wang QY, Han J, Han JH, Shin HE, Ge MH, Park W, Li FY. Exploration of nanozymes in viral diagnosis and therapy. Exploration. 2022;2(1):20210086. https://doi.org/10.1002/exp.20210086.
Yu CJ, Chen TH, Jiang JY, Tseng WL. Lysozyme-directed synthesis of platinum nanoclusters as a mimic oxidase. Nanoscale. 2014;6(16):9618–24. https://doi.org/10.1039/c3nr06896j.
He SB, Wu GW, Deng HH, Liu AL, Lin XH, Xia XH, Chen W. Choline and acetylcholine detection based on peroxidase-like activity and protein antifouling property of platinum nanoparticles in bovine serum albumin scaffold. Biosens Bioelectron. 2014;62:331–6. https://doi.org/10.1016/j.bios.2014.07.005.
Lin XQ, Deng HH, Wu GW, Peng HP, Liu AL, Lin XH, Xia XH, Chen W. Platinum nanoparticles/graphene-oxide hybrid with excellent peroxidase-like activity and its application for cysteine detection. Analyst. 2015;140(15):5251–6. https://doi.org/10.1039/c5an00809c.
Chen WW, Fang X, Li H, Cao HM, Kong JL. DNA-mediated inhibition of peroxidase-like activities on platinum nanoparticles for simple and rapid colorimetric detection of nucleic acids. Biosens Bioelectron. 2017;94:169–75. https://doi.org/10.1016/j.bios.2017.02.025.
Fan J, Yin JJ, Ning B, Wu XC, Hu Y, Ferrari M, Anderson GJ, Wei JY, Zhao Y, Nie GJ. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials. 2011;32(6):1611–8. https://doi.org/10.1016/j.biomaterials.2010.11.004.
Jawaid P, Rehman M, Yoshihisa Y, Li P, Zhao QL, Hassan MA, Miyamoto Y, Shimizu T, Kondo T. Effects of SOD/catalase mimetic platinum nanoparticles on radiation-induced apoptosis in human lymphoma U937 cells. Apoptosis. 2014;19(6):1006–16. https://doi.org/10.1007/s10495-014-0972-5.
Liu Y, Wu HH, Li M, Yin JJ, Nie ZH. pH dependent catalytic activities of platinum nanoparticles with respect to the decomposition of hydrogen peroxide and scavenging of superoxide and singlet oxygen. Nanoscale. 2014;6(20):11904–10. https://doi.org/10.1039/c4nr03848g.
Deng HH, Lin XL, Liu YH, Li KL, Zhuang QQ, Peng HP, Liu AL, Xia XH, Chen W. Chitosan-stabilized platinum nanoparticles as effective oxidase mimics for colorimetric detection of acid phosphatase. Nanoscale. 2017;9(29):10292–300. https://doi.org/10.1039/c7nr03399k.
Weng XL, Lin S, Zhong YH, Chen ZL. Chitosan stabilized bimetallic Fe/Ni nanoparticles used to remove mixed contaminants-amoxicillin and Cd (II) from aqueous solutions. Chem Eng J. 2013;229:27–34. https://doi.org/10.1016/j.cej.2013.05.096.
Hettiarachchi MA, Wickramarachchi PASR. Synthesis of chitosan stabilized silver nanoparticles using gamma ray irradiation and characterization. J Sci Univ Kelaniya Sri Lanka. 2012;6:45–52. https://doi.org/10.4038/josuk.v6i0.4222.
Amarnath K, Kumar J, Reddy T, Mahesh V, Ayyappan SR, Nellore J. Synthesis and characterization of chitosan and grape polyphenols stabilized palladium nanoparticles and their antibacterial activity. Colloid Surface B. 2012;92:254–61. https://doi.org/10.1016/j.colsurfb.2011.11.049.
Deng HH, Lin XL, He SB, Wu GW, Wu WH, Yang Y, Lin Z, Peng HP, Xia XH, Chen W. Colorimetric tyrosinase assay based on catechol inhibition of the oxidase-mimicking activity of chitosan-stabilized platinum nanoparticles. Microchim Acta. 2019;186(5):301. https://doi.org/10.1007/s00604-019-3451-4.
He SB, Yang L, Yang Y, Noreldeen HAA, Wu GW, Peng HP, Deng HH, Chen W. Carboxylated chitosan enabled platinum nanozyme with improved stability and ascorbate oxidase-like activity for a fluorometric acid phosphatase sensor. Carbohydr Polym. 2022;298:120120. https://doi.org/10.1016/j.carbpol.2022.120120.
Zhang BW, Li X, Sun WL, Xing Y, Xiu ZL, Zhuang CL, Dong YS. Dietary flavonoids and acarbose synergistically inhibit alpha-glucosidase and lower postprandial blood glucose. J Agric Food Chem. 2017;65(38):8319–30. https://doi.org/10.1021/acs.jafc.7b02531.
Zhao HH, Liu YQ, Chen J. Screening of α-glucosidase inhibitors from natural flavonoids by an in-capillary assay combining PMMA and EMMA. Anal Methods. 2019;11(10):1371–8. https://doi.org/10.1039/c8ay02232a.
Zhong Y, Li QL, Lu M, Wang T, Yang H, He Q, Cui X, Li X, Zhao S. A colorimetric sensing strategy based on enzyme@metal-organic framework and oxidase-like IrO2/MnO2 nanocomposite for α-glucosidase inhibitor screening. Microchim Acta. 2020;187(12):675. https://doi.org/10.1007/s00604-020-04660-6.
Zhong Y, Yu L, He Q, Zhu Q, Zhang C, Cui X, Zheng J, Zhao S. Bifunctional hybrid enzyme-catalytic metal organic framework reactors for α-glucosidase inhibitor screening. ACS Appl Mater Interfaces. 2019;11(36):32769–77. https://doi.org/10.1021/acsami.9b11754.
Liu JS, Wu FF, Liu CF, Bao HJ, Fu T. “Turn-on” fluorometric probe for alpha-glucosidase activity using red fluorescent carbon dots and 3,3′,5,5′-tetramethylbenzidine. Microchim Acta. 2020;187(9):498. https://doi.org/10.1007/s00604-020-04479-1.
Yuan XC, Sun Y, Zhao PF, Zhao LS, Xiong ZL. Redox-induced target-dependent ratiometric fluorescence sensing strategy and logic gate operation for detection of alpha-glucosidase activity and its inhibitor. Dalton Trans. 2021;50(27):9426–37. https://doi.org/10.1039/d1dt01299a.
Funding
The authors are grateful to the Startup Fund for Scientific Research, Fujian Medical University (2019QH1006 and 2018QH1013) and the Natural Science Foundation of Fujian Province (2022J01271) for the financial support that made this research possible.
Author information
Authors and Affiliations
Contributions
Qin-Qin Yang, Shao-Bin He, and Hao-Hua Deng conceptualized the article. Qin-Qin Yang, Yi-Lin Zhang, and Ming Li conducted the literature search and drafted the manuscript. Xiu-Hua You, Bo-Wen Xiao, Liu Yang, and Zhi-Qiang Yang assisted in conceptualizing and producing the figures and graphical abstract. Wei Chen critically revised the work.
Corresponding authors
Ethics declarations
Ethics approval
The research involved human serum samples taken from healthy volunteers who provided informed written consent to participate in the study. This project was approved by the Biomedical Research Ethics Committee of Fujian Medical University (Approval No. 2022–141). All tests were conducted in accordance with the ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Nanozymes with guest editors Vipul Bansal, Sudipta Seal, and Hui Wei.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, QQ., He, SB., Zhang, YL. et al. A colorimetric sensing strategy based on chitosan-stabilized platinum nanoparticles for quick detection of α-glucosidase activity and inhibitor screening. Anal Bioanal Chem (2024). https://doi.org/10.1007/s00216-024-05198-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00216-024-05198-9