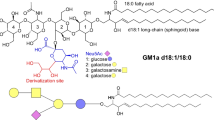
Abstract
Gangliosides play an imperative role in cell signaling, neuronal recovery, apoptosis, and other physiological processes. For example, GM3 can regulate hypothalamic leptin resistance and control energy homeostasis, GD3 can mediate cell proliferation and differentiation and induce apoptosis, and GQ1b can stimulate neurogenesis. Therefore, the present study sought to establish and optimize the targeted analysis method for ganglioside subclasses and their molecular species using hydrophilic interaction liquid chromatography–triple quadrupole–MS/MS (HILIC-QQQ-MS/MS). Additionally, the fragmentation pattern of different ganglioside subclasses and their retention time patterns were analyzed, providing more accurate qualitative results. The limit of quantitation (LOQ) was as low as 10−4 ng. Moreover, the molecular species of gangliosides in the liver, cortex, and hypothalamus of C57BL/6 mice were analyzed using the established method. A total of 23 ganglioside subclasses with 164 molecular species, including 40 O-acetylated ganglioside molecular species and 28 NeuGc ganglioside molecular species, were identified using the semi-quantitative analysis method of an external standard curve corrected by an internal standard. In addition to NeuGc gangliosides, the contents of ganglioside subclasses were more abundant in the mouse brain than those in the mouse liver; especially, the contents of unsaturated gangliosides in the hypothalamus were much higher than those in the liver. Among them, O-acetylated gangliosides were detected only in the cortex and hypothalamus at a concentration of up to 100 μg/mg protein (40 molecular species). Overall, the proposed method expanded the detectable number of ganglioside subclasses and molecular species in biological samples and provided more opportunities for further study of the biological functions of gangliosides.






Similar content being viewed by others
Abbreviations
- Cer:
-
Ceramide
- Glc:
-
D-glucose
- Gal:
-
D-galactose
- GalNAc:
-
N-acetylgalactosamine
- Fuc:
-
Fucose
- NeuAc:
-
N-acetylneuraminic acid
- NeuGc:
-
N-glycolylneuraminic acid
- O-Ac:
-
O-Acetylated
- GC-MS:
-
Gas chromatography–mass spectrometry
- AP-MALDI-MS:
-
Atmospheric pressure matrix-assisted laser desorption ionization mass spectrometry
- HILIC-ESI–MS/MS:
-
Hydrophilic interaction liquid chromatography–electrospray ionization coupled with mass spectrometry
- MRM:
-
Multi-stage reaction monitoring
- QQQ:
-
Triple quadrupole
- QC:
-
Quality control
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
References
Schengrund CL. Gangliosides: glycosphingolipids essential for normal neural development and function. Trends Biochem Sci. 2015;40:397–406. https://doi.org/10.1016/j.tibs.2015.03.007.
Svennerholm L. Designation and schematic structure of gangliosides and allied glycosphingolipids. Prog Brain Res. 1994;101:11–4. https://doi.org/10.1016/S0079-6123(08)61935-4.
Breiden B, Sandhoff K. Ganglioside metabolism and its inherited diseases. Gangliosides: Methods and Protocols. 2018;1804:97–141. https://doi.org/10.1007/978-1-4939-8552-4_5.
Schnaar RL, GerardySchahn R, Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518. https://doi.org/10.1152/physrev.00033.2013.
Schwarzkopf M, Knobeloch KP, Rohde E, Hinderlich S, Wiechens N, Lucka L, Horak I, Reutter W, Horstkorte R. Sialylation is essential for early development in mice. Proc Natl Acad Sci. 2002;99:5267–70. https://doi.org/10.1073/pnas.072066199.
Inokuchi J, Kanoh H, Inamori K, Nagafuku M, Nitta T, Fukase K. Homeostatic and pathogenic roles of the GM3 ganglioside. FEBS J. 2022;289:5152–65. https://doi.org/10.1111/febs.16076.
Zhang ZH, Liu WH, Shen ML, Ma XY, Li RQ, Di JX, Bai H, Gao L. Protective effect of GM1 attenuates hippocampus and cortex apoptosis after ketamine exposure in neonatal rat via PI3K/AKT/GSK3β pathway. Mol Neurobiol. 2021;58:3471–83. https://doi.org/10.1007/s12035-021-02346-5.
Karpiak SE. Exogenous gangliosides enhance recovery from CNS injury. Adv Exp Med Biol. 1984;174:489–97. https://doi.org/10.1007/978-1-4684-1200-0_41.
Fujimoto Y, Izumoto S, Suzuki T, Kinoshita M, Kagawa N, Wada K, Hashimoto N, Maruno M, Nakatsuji Y, Yoshimine T. Ganglioside GM3 inhibits proliferation and invasion of glioma. J Neurooncol. 2005;71:99–106. https://doi.org/10.1007/s11060-004-9602-3.
Fabris D, Karmelić I, Muharemović H, Sajko T, Jurilj M, Potočki S, Novak R, Vukelić Ž. Ganglioside composition distinguishes anaplastic ganglioglioma tumor tissue from peritumoral brain tissue: complementary mass spectrometry and thin-layer chromatography evidence. Int J Mol Sci. 2021;22:8844. https://doi.org/10.3390/ijms22168844.
Arends M, Weber M, Papan C, Damm M, Surma MA, Spiegel C, Djannatian M, Li S, Connell L, Johannes L, Schifferer M, Klose C, Simons M. Ganglioside lipidomics of CNS myelination using direct infusion shotgun mass spectrometry. iScience. 2022;25:105323. https://doi.org/10.1016/j.isci.2022.105323.
Li ZC, Bin ZQ. Ganglioside isomer analysis using ion polarity switching liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2021;413:3269–79. https://doi.org/10.1007/s00216-021-03262-2.
Yamaguchi Y, Yamaguchi T, Kato K. Structural analysis of oligosaccharides and glycoconjugates using NMR. Adv Neurobiol. 2023;29:165–83. https://doi.org/10.1007/978-3-031-12390-0_6.
Viljetić B, Labak I, Majić S, Štambuk A, Heffer M. Distribution of mono-, di- and trisialo gangliosides in the brain of Actinopterygian fishes. Biochim Biophys Acta (BBA) - General Subjects. 2012;1820:1437–43. https://doi.org/10.1016/j.bbagen.2011.12.010.
Walworth MJ, Stankovich JJ, Van Berkel GJ, Schulz M, Minarik S, Nichols J, Reich E. Hydrophobic treatment enabling analysis of wettable surfaces using a liquid microjunction surface sampling probe/electrospray ionization-mass spectrometry system. Anal Chem. 2011;83:591–7. https://doi.org/10.1021/ac102634e.
Cameron SJS, Lewis KE, Beckmann M, Allison GG, Ghosal R, Lewis PD, Mur LAJ. The metabolomic detection of lung cancer biomarkers in sputum. Lung Cancer. 2016;94:88–95. https://doi.org/10.1016/j.lungcan.2016.02.006.
Zhang YY, Wang J, Liu JA, Han JJ, Xiong SX, Yong WD, Zhao ZW. Combination of ESI and MALDI mass spectrometry for qualitative, semi-quantitative and in situ analysis of gangliosides in brain. Sci Rep. 2016;6:25289. https://doi.org/10.1038/srep25289.
Hořejší K, Jin C, Vaňková Z, Jirásko R, Strouhal O, Melichar B, Teneberg S, Holčapek M. Comprehensive characterization of complex glycosphingolipids in human pancreatic cancer tissues. J Biol Chem. 2023;299: 102923. https://doi.org/10.1016/j.jbc.2023.102923.
Masson EY, Sibille E, Martine L, ChauxPicquet F, Bretillon L, Berdeaux O. Apprehending ganglioside diversity: a comprehensive methodological approach. J Lipid Res. 2015;56:1821–35. https://doi.org/10.1194/jlr.D060764.
Fong BY, Ma L, Khor GL, van der Does Y, Rowan A, McJarrow P, MacGibbon AKH. Ganglioside composition in beef, chicken, pork, and fish determined using liquid chromatography–high-resolution mass spectrometry. J Agric Food Chem. 2016;64:6295–305. https://doi.org/10.1021/acs.jafc.6b02200.
Ren TK, Li ML, Zheng H, Wang Z, Zhang JL. Characterization of acidic glycosphingolipid changes in C6 glioma rats treated with temozolomide using ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Anal Test. 2020;4:217–25. https://doi.org/10.1007/s41664-020-00140-1.
Fong B, Norris C, Lowe E, McJarrow P. Liquid chromatography-high-resolution mass spectrometry for quantitative analysis of gangliosides. Lipids. 2009;44:867–74. https://doi.org/10.1007/s11745-009-3327-1.
Ma YX, Wang XC, Wang ZG, Cong PX, Xu J, Xue CH. Characterization of gangliosides in three sea urchin species by HILIC–ESI-MS/MS. J Agric Food Chem. 2021;69:7641–51. https://doi.org/10.1021/acs.jafc.1c02058.
Svennerholm L, Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta (BBA) - Lipids and Lipid Metabolism. 1980;617:97–109. https://doi.org/10.1016/0005-2760(80)90227-1.
Hájek R, Jirásko R, Lísa M, Cífková E, Holčapek M. Hydrophilic interaction liquid chromatography–mass spectrometry characterization of gangliosides in biological samples. Anal Chem. 2017;89:12425–32. https://doi.org/10.1021/acs.analchem.7b03523.
Konermann L. Addressing a common misconception: ammonium acetate as neutral pH “buffer” for native electrospray mass spectrometry. J Am Soc Mass Spectrom. 2017;28:1827–35. https://doi.org/10.1007/s13361-017-1739-3.
Weng ND. Bioanalytical liquid chromatography tandem mass spectrometry methods on underivatized silica columns with aqueous/organic mobile phases. J Chromatogr B. 2003;796:209–24. https://doi.org/10.1016/j.jchromb.2003.08.026.
Li H, Xu RL, Yang LJ, Luan HM, Chen SL, Chen L, Cai ZW, Tian RJ. Combinatory data-independent acquisition and parallel reaction monitoring method for deep profiling of gangliosides. Anal Chem. 2020;92:10830–8. https://doi.org/10.1021/acs.analchem.0c02313.
Meng XY, Yau LF, Huang H, Chan WH, Luo P, Chen L, Tong TT, Mi JN, Yang ZF, Jiang ZH, Wang JR. Improved approach for comprehensive profiling of gangliosides and sulfatides in rat brain tissues by using UHPLC-Q-TOF-MS. Chem Phys Lipids. 2019;225: 104813. https://doi.org/10.1016/j.chemphyslip.2019.104813.
Cavdarli S, Delannoy P, Groux-Degroote S. O-acetylated gangliosides as targets for cancer immunotherapy. Cells. 2020;9:741. https://doi.org/10.3390/cells9030741.
Chan WH, Yau LF, Meng XY, Chan KM, Jiang ZH, Wang JR. Robust quantitation of gangliosides and sulfatides in human brain using UHPLC-MRM-MS: method development and application in Alzheimer’s disease. Talanta. 2023;256: 124264. https://doi.org/10.1016/J.TALANTA.2023.124264.
Cavdarli S, Yamakawa N, Clarisse C, Aoki K, Brysbaert G, Le Doussal JM, Delannoy P, Guérardel Y, Groux-Degroote S. Profiling of o-acetylated gangliosides expressed in neuroectoderm derived cells. Int J Mol Sci. 2020;21:370. https://doi.org/10.3390/ijms21010370.
Kyoko N, Masaru N, Michiko S, Michihiro I, Toshio A, Tetsuji A, Tadashi M, Akemi S, Tamio Y. Gangliosides of hog skeletal muscle. Biochim Biophys Acta (BBA)/Lipids and Lipid Metabolism. 1983;752:291–300. https://doi.org/10.1016/0005-2760(83)90126-1.
Nakamura K, Ariga T, Yahagi T, Miyatake T, Suzuki A, Yamakawa T. Interspecies comparison of muscle gangliosides by two-dimensional thin-layer chromatography. J Biochem. 1983;94:1359–65. https://doi.org/10.1093/oxfordjournals.jbchem.a134482.
Saito M, Kitamura H, Sugiyama K. Liver gangliosides of various animals ranging from fish to mammalian species. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:747–58. https://doi.org/10.1016/S1096-4959(01)00379-7.
Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol. 2018;19:281–96. https://doi.org/10.1038/nrm.2017.138.
Ikeda K, Shimizu T, Taguchi R. Targeted analysis of ganglioside and sulfatide molecular species by LC/ESI-MS/MS with theoretically expanded multiple reaction monitoring. J Lipid Res. 2008;49:2678–89. https://doi.org/10.1194/jlr.D800038-JLR200.
Wormwood Moser KL, Van Aken G, DeBord D, Hatcher NG, Maxon L, Sherman M, Yao L, Ekroos K. High-defined quantitative snapshots of the ganglioside lipidome using high resolution ion mobility SLIM assisted shotgun lipidomics. Anal Chim Acta. 2021;1146:77–87. https://doi.org/10.1016/j.aca.2020.12.022.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities of China (No. 202261047 and No. 202012018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The animal experiment involved in our investigation was approved and conducted following the Animal Ethics Committee of the Ocean University of China (Approval No. SPXY2022062801).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection New Trends in Lipidomics with guest editor Michal Holčapek.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, S., Ma, Y., Song, Y. et al. Establishment of a targeted analysis method for gangliosides in mouse tissues by HILIC-ESI–MS/MS. Anal Bioanal Chem (2024). https://doi.org/10.1007/s00216-024-05169-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00216-024-05169-0