Abstract
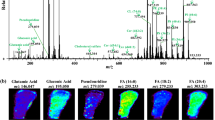
Methionine and choline both are essential nutrients which are needed for methyl group metabolism. A methionine-choline-deficient (MCD) diet leads to pathological changes in the kidney. The mechanism of the MCD diet is complex, and fundamental research is still required to provide a better understanding of the driving forces behind it. We evaluated the regional effects of the MCD diet on the metabolites of mouse kidney tissue using desorption electrospray ionization mass spectrometry imaging technology. A total of 20, 17, and 13 metabolites were significantly changed in the cortex, outer medulla, and inner medulla, respectively, of the mouse kidney tissue after the administration of the MCD diet. Among the discriminating metabolites, only three metabolites (guanidoacetic acid, serine, and nicotinamide riboside) were significantly increased, and all the other metabolites showed a significant decrease. The results showed that there were significant region-specific changes in the serine metabolism, carnitine metabolism, choline metabolism, and arginine metabolism. This study presents unique regional metabolic data, providing a more comprehensive understanding of the molecular characteristics of the MCD diet in the kidney.
Graphical Abstract









Similar content being viewed by others
Abbreviations
- MCD:
-
Methionine-choline-deficient
- MSI:
-
Mass spectrometry imaging
- DESI-MSI:
-
Desorption electrospray ionization mass spectrometry imaging
- H&E:
-
Hematoxylin and eosin
- GPC:
-
Glycerophosphocholine
References
Marí M, Colell A, Morales A, Von Montfort C, Garcia-Ruiz C, Fernández-Checa JC. Redox control of liver function in health and disease. Antioxid Redox Signal. 2010;12:1295–331. https://doi.org/10.1089/ars.2009.2634.
Qasem H, Al-Ayadhi L, Al Dera H, El-Ansary A. Increase of cytosolic phospholipase A2 as hydrolytic enzyme of phospholipids and autism cognitive, social and sensory dysfunction severity. Lipids Health Dis. 2017;16:117. https://doi.org/10.1186/s12944-016-0391-4.
Hamlin JC, Pauly M, Melnyk S, Pavliv O, Starrett W, Crook TA, et al. Dietary intake and plasma levels of choline and betaine in children with autism spectrum disorders. Autism Res Treat. 2013;2013:578429. https://doi.org/10.1155/2013/578429.
Veskovic M, Mladenovic D, Jorgacevic B, Stevanovic I, Luka S, Radosavljevic T. Alpha-lipoic acid affects the oxidative stress in various brain structures in mice with methionine and choline deficiency. Exp Biol Med. 2015;240:418–25. https://doi.org/10.1177/153537021454952.
Zeisel SH, Dacosta KA, Franklin PD, Alexander AE, Lamont JT, Sheard NF, et al. Choline, an essential nutrient for humans. Faseb J. 1991;5:2093–8. https://doi.org/10.1096/fasebj.5.7.2010061.
Ossani G, Dalghi M, Repetto M. Oxidative damage lipid peroxidation in the kidney of choline-deficient rats. Front Biosci. 2007;12:1174–83. https://doi.org/10.2741/2135.
Ossani GP, Repetto MG, Boveris A, Monserrat AJ. The protective effect of menhaden oil in the oxidative damage and renal necrosis due to dietary choline deficiency. Food Funct. 2013;4:448–52. https://doi.org/10.1039/C2FO30229B.
Laho T, Clarke JD, Dzierlenga AL, Li H, Klein DM, Goedken M, et al. Effect of nonalcoholic steatohepatitis on renal filtration and secretion of adefovir. Biochem Pharmacol. 2016;115:144–51. https://doi.org/10.1016/j.bcp.2016.07.001.
Gong M, Lu H, Li L, Feng M, Zou Z. Integration of transcriptomics and metabonomics revealed the protective effects of hemp seed oil against methionine–choline-deficient diet-induced non-alcoholic steatohepatitis in mice. Food Funct. 2023;14:2096–111. https://doi.org/10.1039/D2FO03054C.
He D, Su Y, Meng D, Wang X, Wang J, Ye H. A pilot study optimizing metabolomic and lipidomic acquisition in serum for biomarker discovery in nonalcoholic fatty liver disease. J Mass Spectrom Adv Clin Lab. 2021;22:17–25. https://doi.org/10.1016/j.jmsacl.2021.10.001.
Lim YJ, Tonial NC, Hartjes ED, Haig A, Velenosi TJ, Urquhart BL. Metabolomics for the identification of early biomarkers of nephrotoxicity in a mouse model of cisplatin-induced acute kidney injury. Biomed Pharmacother. 2023;163:114787. https://doi.org/10.1016/j.biopha.2023.114787.
Bai LN, Wang YZ, Zhang L, Liu YY, Chen SJ, Xie XM, et al. In situ metabolomics of metabolic reprogramming involved in a mouse model of type 2 diabetic kidney disease. Front Physiol. 2021;12:779683. https://doi.org/10.3389/fphys.2021.779683.
Bergman HM, Lindfors L, Palm F, Kihlberg J, Lanekoff I. Metabolite aberrations in early diabetes detected in rat kidney using mass spectrometry imaging. Anal Bioanal Chem. 2019;411:2809–16. https://doi.org/10.1007/s00216-019-01721-5.
Zhang JL, Li SQ, Lin JQ, Yu WD, Eberlin LS. Mass spectrometry imaging enables discrimination of renal oncocytoma from renal cell cancer subtypes and normal kidney tissues. Cancer Res. 2020;80:689–98. https://doi.org/10.1158/0008-5472.CAN-19-2522.
Zhang X, Liu Y, Yang S, Gao X, Wang S, Wang Z, et al. Comparison of local metabolic changes in diabetic rodent kidneys using mass spectrometry imaging. Metabolites. 2023;13:324. https://doi.org/10.3390/metabo13030324.
Wiseman JM, Ifa DR, Zhu YX, Kissinger CB, Manicke NE, Kissinger PT, et al. Desorption electrospray ionization mass spectrometry imaging drugs and metabolites in tissues. Proc Natl Acad Sci USA. 2008;105:18120–5. https://doi.org/10.1073/pnas.0801066105.
Ellis SR, Wu CP, Deeley JM, Zhu XJ, Truscott RJW, Panhuis MIH, et al. Imaging of human lens lipids by desorption electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2010;21:2095–104. https://doi.org/10.1016/j.jasms.2010.09.003.
Wang X, Hou Y, Hou Z, Xiong W, Huang G. Mass spectrometry imaging of brain cholesterol and metabolites with trifluoroacetic acid-enhanced desorption electrospray ionization. Anal Chem. 2019;91:2719–26. https://doi.org/10.1021/acs.analchem.8b04395.
Wang X, Yang X, Hou Z, Tian S, Xu G, Li J, et al. Whole-brain mapping of metabolic alterations in a mouse model of Alzheimer’s disease by desorption electrospray ionization mass spectrometry imaging. Talanta. 2023;253:124046. https://doi.org/10.1016/j.talanta.2022.124046.
Tillner J, McKenzie JS, Jones EA, Speller AVM, Walsh JL, Veselkov KA, et al. Investigation of the impact of desorption electrospray ionization sprayer geometry on its performance in imaging of biological tissue. Anal Chem. 2016;88:4808–16. https://doi.org/10.1021/acs.analchem.6b00345.
Sogaard SB, Andersen SB, Taghavi I, Hoyos CAV, Christoffersen C, Hansen KL, et al. Super-resolution ultrasound imaging provides quantification of the renal cortical and medullary vasculature in obese Zucker rats: a pilot study. Diagnostics. 2022;12:1626. https://doi.org/10.3390/diagnostics12071626.
Boch J, Kempf B, Bremer E. Osmoregulation in bacillus-subtilis-synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol. 1994;176:5364–71. https://doi.org/10.1128/jb.176.17.5364-5371.1994.
Ilozumba MN, Cheng TYD, Neuhouser ML, Miller JW, Beresford SAA, Duggan DJ, et al. Associations between plasma choline metabolites and genetic polymorphisms in one-carbon metabolism in postmenopausal women: the women’s health initiative observational study. J Nutr. 2020;150:2874–81. https://doi.org/10.1093/jn/nxaa266.
Ahmad NA, Raizman M, Weizmann N, Wasek B, Arning E, Bottiglieri T, et al. Betaine attenuates pathology by stimulating lipid oxidation in liver and regulating phospholipid metabolism in brain of methioninecholine–deficient rats. 2019;33:9334–49. https://doi.org/10.1096/fj.201802683R
Penry JT, Manore MM. Choline: an important micronutrient for maximal endurance-exercise performance? Int J Sport Nutr Exerc Metab. 2008;18:191–203. https://doi.org/10.1123/ijsnem.18.2.191.
Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis. 2011;34:3–15. https://doi.org/10.1007/s10545-010-9088-4.
Bernhard W, Poets CF, Franz AR. Choline and choline-related nutrients in regular and preterm infant growth. Eur J Nutr. 2019;58:931–45. https://doi.org/10.1007/s00394-018-1834-7.
Bieber LL. Carnitine. Ann Rev Biochem. 1988;57:261–83. https://doi.org/10.1146/annurev.bi.57.070188.001401.
Zahedi E, Sadr S, Sanaeierad A, Roghani M. Chronic acetyl-L-carnitine treatment alleviates behavioral deficits and neuroinflammation through enhancing microbiota derived-SCFA in valproate model of autism. Biomed Pharmacother. 2023;163:114848. https://doi.org/10.1016/j.biopha.2023.114848.
Merritt JL II, Norris M, Kanungo S. Fatty acid oxidation disorders. Ann Transl Med. 2018;6:473. https://doi.org/10.21037/atm.2018.10.57.
Li WW, Ma FF, Zhang LY, Huang Y, Li XH, Zhang AJ, et al. S-propargyl-cysteine exerts a novel protective effect on methionine and choline deficient diet-induced fatty liver via akt/Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2016;2016:4690857. https://doi.org/10.1155/2016/4690857.
Ferraretto A, Bottani M, Villa I, Giusto L, Signo M, Senesi P, et al. L-carnitine activates calcium signaling in human osteoblasts. J Funct Foods. 2018;47:270–8. https://doi.org/10.1016/j.jff.2018.05.068.
Strilakou AA, Lazaris AC, Perelas AI, Mourouzis IS, Douzis IC, Karkalousos PL, et al. Heart dysfunction induced by choline-deficiency in adult rats: the protective role of L-carnitine. Eur J Pharmacol. 2013;709:20–7. https://doi.org/10.1016/j.ejphar.2013.03.025.
Mollica G, Senesi P, Codella R, Vacante F, Montesano A, Luzi L, et al. L-carnitine supplementation attenuates NAFLD progression and cardiac dysfunction in a mouse model fed with methionine and choline-deficient diet. Dig Liver Dis. 2020;52:314–23. https://doi.org/10.1016/j.dld.2019.09.002.
Gnoni A, Longo S, Gnoni GV, Giudetti AM. Carnitine in human muscle bioenergetics: can carnitine supplementation improve physical exercise? Molecules. 2020;25:182. https://doi.org/10.3390/molecules25010182.
Peng KY, Watt MJ, Rensen S, Greve JW, Huynh K, Jayawardana KS, et al. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J Lipid Res. 2018;59:1977–86. https://doi.org/10.1194/jlr.M085613.
de Miranda JL, Rodrigues BL, de Moura LC, da Rocha GS, Oliveira SD. Versatility of guanidoacetic acid coordination modes and synthesis of its new zinc complex. Results Chem. 2023;5:100785. https://doi.org/10.1016/j.rechem.2023.100785.
Levillain O, Marescau B, Possemiers I, De Deyn PP. Accumulation of methylguanidine and changes in guanidino compound levels in plasma, urine, and kidneys of furosemide-treated rats. Metabolism. 2008;57:802–10. https://doi.org/10.1016/j.metabol.2008.01.025.
Ostojic SM. Guanidinoacetic acid as a performance-enhancing agent. Amino Acids. 2015;48:1867–75. https://doi.org/10.1007/s00726-015-2106-y.
Acknowledgements
We thank Professor Guangming Huang (University of Science and Technology of China) for the provision of Exactive Plus mass spectrometer and DESI platform.
Funding
This research was supported by the Anhui University of Chinese Medicine Talent Support Program (No.: 2022rcyb009), Anhui Provincial Natural Science Foundation (No.: 2108085J45), and Natural Science Research Project of Anhui Educational Committee (No.: 2022AH050498).
Author information
Authors and Affiliations
Contributions
XW: conceptualization, methodology, software, writing—original draft, data curation, investigation, project administration, visualization. YH: conceptualization, methodology, investigation, resources. WZ: conceptualization, methodology, resources. DW: resources, supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Ethics approval
The use of laboratory animals and all study protocols were approved by the Animal Protection Committee and Use Committee of Anhui University of Chinese Medicine.
Source of biological material
Male C57BL/6J mice were obtained from Jiangsu Huachuang Sino Pharmatech Co., Ltd..
Statement on animal welfare
The management and use of animals during the experiment comply with the relevant regulations of “Guide for the Care and Use of Laboratory Animals issued by the National Research Council of the United States (2010),” “Regulations for the administration of affairs concerning laboratory animals (2017)” issued by the Science and Technology Commission of China, and the relevant regulations provided by the Animal Protection Committee and Use Committee of Anhui University of Chinese Medicine.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Hu, Y., Zhu, W. et al. Investigation of metabolite alterations in the kidneys of methionine-choline-deficient mouse by mass spectrometry imaging. Anal Bioanal Chem 416, 1011–1022 (2024). https://doi.org/10.1007/s00216-023-05091-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05091-x