Abstract
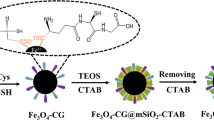
Novel hydrophilic poly(N, N-methylenebisacrylamide/1,2-epoxy-5-hexene) coated magnetic nanospheres functionalized with 2-aminopurine (denoted as Fe3O4@poly(MBA/EH)@2AP) for enriching glycopeptides and glycosylated exosomes were successfully obtained using a simple and green method on the basis of the HILIC (hydrophilic interaction liquid chromatography) enrichment strategy. The high density of polar groups endows the material with amazing hydrophilicity, enabling the nanomaterial to successfully capture glycopeptides and glycosylated exosomes within 1 min. Meanwhile, the materials demonstrated great sensitivity (0.01 fmol/μL), good loading capability (125 μg/mg), high selectivity (BSA:HRP = 1000:1), and repeatability (more than 10 times). Besides, the material was applied in the analysis of bio-samples, a total of 290 glycosylated peptides and 184 glycosylation sites mapping to 185 glycoproteins were identified in the serum of uremic patients. Besides, 42 glycopeptides were enriched from the saliva of healthy people. At the same time, it was verified by TEM and western blot that the complete glycosylated exosomes were successfully captured from the serum of the uremic patients. All experiments have demonstrated that Fe3O4@poly(MBA/EH)@2AP has a promising future in practical applications.
Graphical Abstract










Similar content being viewed by others
References
Martins TS, Vaz M, Henriques AG. A review on comparative studies addressing exosome isolation methods from body fluids. Anal Bioanal Chem. 2022;415:1239–63. https://doi.org/10.1007/s00216-022-04174-5.
Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Kyakulaga AH, Wilcher SA, Gupta RC. Milk exosomes - natural nanoparticles for siRNA delivery. Cancer Lett. 2019;449:186–95. https://doi.org/10.1016/j.canlet.2019.02.011.
Konoshenko M, Sagaradze G, Orlova E, Shtam T, Proskura K, Kamyshinsky R, Yunusova N, Alexandrova A, Efimenko A, Tamkovich S. Total blood exosomes in breast cancer: potential role in crucial steps of tumorigenesis. Int J Mol Sci. 2020;21(19):7341. https://doi.org/10.3390/ijms21197341.
Zhang N, Hu XF, Chen HL, Deng CH, Sun NR. Specific enrichment and glycosylation discrepancy profiling of cellular exosomes using a dual-affinity probe. Chem Commun. 2021;57(51):6249–52. https://doi.org/10.1039/d1cc01530c.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. https://doi.org/10.1038/s41556-018-0250-9.
Zhao XX, Zhang WQ, Qiu XP, Mei Q, Luo Y, Fu WL. Rapid and sensitive exosome detection with CRISPR/Cas12a. Anal Bioanal Chem. 2020;412:601–9. https://doi.org/10.1007/s00216-019-02211-4.
Hsu MT, Wang YK, Tseng YJ. Exosomal proteins and lipids as potential biomarkers for lung cancer diagnosis, prognosis, and treatment. Cancers. 2022;12:732. https://doi.org/10.3390/cancers14030732.
Chen YF, Ma XY, Lou CT, Zhou CW, Zhao XD, Li N, Tian HH, Meng XD. PLA2G10 incorporated in exosomes could be diagnostic and prognostic biomarker for non-small cell lung cancer. Clin Chim Acta. 2022;530:55–65. https://doi.org/10.1016/j.cca.2022.02.016.
Xu K, Jin YL, Li YM, Huang YY, Zhao R. Recent progress of exosome isolation and peptide recognition-guided strategies for exosome research. Front Chem. 2022;10:844124. https://doi.org/10.3389/fchem.2022.844124.
Chen JC, Li PL, Zhang TY, Xu ZP, Huang XW, Wang RM, Du LT. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2022;9:811971. https://doi.org/10.3389/fbioe.2021.811971.
Shao HL, Im H, Castro CM, Breakefield X, Weissleder R, Lee HH. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–50. https://doi.org/10.1021/acs.chemrev.7b00534.
Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol. 2020;21:729–49. https://doi.org/10.1038/s41580-020-00294-x.
Lu Q, Chen C, Xiong YT, Li GD, Zhang XF, Zhang YH, Wang DD, Zhu ZC, Li XL, Qing GY, Sun TL, Liang XM. High-efficiency phosphopeptide and glycopeptide simultaneous enrichment by hydrogen bond-based bifunctional smart polymer. Anal Chem. 2020;92:6269–77. https://doi.org/10.1021/acs.analchem.9b02643.
Bindeman WE, Fingleton B. Glycosylation as a regulator of site-specific metastasis. 2022;41:107–29. https://doi.org/10.1007/s10555-021-1001.
Suttapitugsakul S, Sun FX, Wu RH. Recent advances in glycoproteomic analysis by mass spectrometry. Anal Chem. 2020;92:267–91. https://doi.org/10.1021/acs.analchem.9b04651.
Wang MM, Zhu JH, Lubman DM, Gao CF. Aberrant glycosylation and cancer biomarker discovery: a promising and thorny journey. Clin Chem Lab Med. 2019;57:407–16. https://doi.org/10.1515/cclm-2018-0379.
Kim KH, Park GW, Jeong JE, Ji ES, An HJ, Kim JY, Yoo JS. Parallel reaction monitoring with multiplex immunoprecipitation of N-glycoproteins in human serum for detection of hepatocellular carcinoma. Anal Bioanal Chem. 2019;411:3009–19. https://doi.org/10.1007/s00216-019-01775-5.
Nunez C. Blood-based protein biomarkers in breast cancer. Clin Chim Acta. 2019;90:113–27. https://doi.org/10.1016/j.cca.2018.12.028.
Brandi J, Noberini R, Bonaldi T, Cecconi D. Advances in enrichment methods for mass spectrometry-based proteomics analysis of post-translational modifications. J Chromatogr A. 2022;1678:463352. https://doi.org/10.1016/j.chroma.2022.463352.
Yin HD, Zhu JH. Methods for quantification of glycopeptides by liquid separation and mass spectrometry. Mass Spectrom Rev. 2022;42:887–917. https://doi.org/10.1002/mas.21771.
Sun NR, Yu HL, Wu H, Shen XZ, Deng CH. Advanced nanomaterials as sample technique for bio-analysis. Trac-Trends Anal Chem. 2021;135:37. https://doi.org/10.1016/j.trac.2020.116168.
Xiao HP, Sun FX, Suttapitugsakul S, Wu RH. Global and site-specific analysis of protein glycosylation in complex biological systems with mass spectrometry. Mass Spectrom Rev. 2019;38:356–79. https://doi.org/10.1002/mas.21586.
Cerrato A, Cavaliere C, Montone CM, Piovesana S. Anal Chim Acta. 2023;1245:340862. https://doi.org/10.1016/j.aca.2023.340862.
Luo B, Chen Q, He J, Li ZY, Yu LZ, Lan F, Wu Y. Boronic acid-functionalized magnetic metal-organic frameworks via a dual-ligand strategy for highly efficient enrichment of Phosphopeptides and Glycopeptides. ACS Sustain Chem Eng. 2019;7(6):6043–52. https://doi.org/10.1021/acssuschemeng.8b06171.
Welch CJ, Talaga ML, Kadav PD, Edwards JL, Bandyopadhyay P, Dam TK. A capture and release method based on noncovalent ligand cross-linking and facile filtration for purification of lectins and glycoproteins. J Biol Chem. 2020;295(1):223–36. https://doi.org/10.1074/jbc.RA119.010625.
Sajid MS, Jabeen F, Najam-ul-Haq M. Hydrazide-functionalized affinity on conventional support materials for glycopeptide enrichment. Anal Bioanal Chem. 2017;409:3135–43. https://doi.org/10.1007/s00216-017-0254-5.
Zhao Y, Raidas S, Mao Y, Li N. Glycine additive facilitates site-specific glycosylation profiling of biopharmaceuticals by ion-pairing hydrophilic interaction chromatography mass spectrometry. Anal Bioanal Chem. 2021;413:1267–77. https://doi.org/10.1007/s00216-020-03089-3.
Yue XY, Qin HQ, Chen Y, Fang Z, Liu LY, Zhu H, Liu XY, Zhou JH, Tian KL, Qiao XQ, Ye ML. Highly efficient enrichment of O-GalNAc glycopeptides by using immobilized metal ion affinity Chromatography. Anal Chem. 2021;93:7579–87. https://doi.org/10.1021/acs.analchem.0c05236.
Li Y, Xu JW, Li XW, Ma SJ, Wei YM, Ou JJ. One-step fabrication of nitrogen-rich linear porous organic polymer-based micron-sized sphere for selective enrichment of glycopeptides. Anal Chim Acta. 2022;1215:339988. https://doi.org/10.1016/j.aca.2022.339988.
Hemstrom P, Irgum K. Hydrophilic interaction chromatography. J Sep Sci. 2006;29(12):1784–821. https://doi.org/10.1002/jssc.200600199.
Sheng QY, Li JY, Chen YX, Liang XM, Lan MB. Hydrophilic graphene oxide-dopamine-cationic cellulose composites and their applications in N-Glycopeptides enrichment. Talanta. 2021;226:122112. https://doi.org/10.1016/j.talanta.2021.122112.
Tian Y, Tang RZ, Wang X, Zhou JH, Li XW, Ma SJ, Gong BL, Ou JJ. Bioinspired dandelion-like silica nanoparticles modified with L-glutathione for highly efficient enrichment of N-glycopeptides in biological samples. Anal Chim Acta. 2021;1173:338694. https://doi.org/10.1016/j.aca.2021.338694.
Liu QJ, Deng CH, Sun NR. Hydrophilic tripeptide-functionalized magnetic metal-organic frameworks for the highly efficient enrichment of N-linked glycopeptides. Nanoscale. 2018;10:12149–55. https://doi.org/10.1039/C8NR03174F.
Li YL, Wang JW, Sun NR, Deng CH. Glucose-6-phosphate-functionalized magnetic microsphere as novel hydrophilic probe for specific capture of N-linked glycopeptides. Anal Chem. 2017;89:11151–8. https://doi.org/10.1021/acs.analchem.7b03708.
Zhou YY, Xu Y, Zhang CC, Emmer A, Zheng HQ. Amino acid-functionalized two-dimensional hollow cobalt sulfide nanoleaves for the highly selective enrichment of N-linked glycopeptides. Anal Chem. 2020;92:2151–8. https://doi.org/10.1021/acs.analchem.9b04740.
Liu QJ, Xie YQ, Deng CH, Lie Y. One-step synthesis of carboxyl-functionalized metal-organic framework with binary ligands for highly selective enrichment of N-linked glycopeptides. Talanta. 2017;175:477–82. https://doi.org/10.1016/j.talanta.2017.07.067.
Zhang N, Dong J, Li XW, Wang SY, Ou JJ, Ye ML. One-step synthesis of hydrophilic microspheres for highly selective enrichment of N-linked glycopeptides. Anal Chim Acta. 2020;1130:91–9. https://doi.org/10.1016/j.aca.2020.07.049.
Li JN, Wang FJ, Wan H, Liu J, Liu ZY, Cheng K, Zou HF. Magnetic nanoparticles coated with maltose-functionalized polyethyleneimine for highly efficient enrichment of N-glycopeptides. J Chromatogr A. 2015;1425:213–20. https://doi.org/10.1016/j.chroma.2015.11.044.
Sahiner N, Demirci S. Poly ionic liquid cryogel of polyethyleneimine: synthesis, characterization, and testing in absorption studies. J Appl Polym Sci. 2016;133:43478. https://doi.org/10.1002/app.43478.
Chu HM, Zheng HY, Yao JZ, Sun NR, Yan GQ, Deng CH. Magnetic metal phenolic networks: expanding the application of a promising nanoprobe to phosphoproteomics research. Chem Commun. 2020;56:11299–302. https://doi.org/10.1039/d0cc04615a.
Sajid MS, Jovcevski B, Pukala TL, Jabeen F, Najam-ul-Haq M. Fabrication of piperazine functionalized polymeric monolithic tip for rapid enrichment of glycopeptides/glycans. Anal Chem. 2020;92:683–9. https://doi.org/10.1021/acs.analchem.9b02068.
Gao Z, Tang RZ, Ma SJ, Jia SC, Zhang S, Gong BL, Ou JJ. Design and construction of a hydrophilic coating on macroporous adsorbent resins for enrichment of glycopeptides. Anal Methods. 2021;13:4515. https://doi.org/10.1039/d1ay01276b.
Zhang XY, Feng QS, Xie ZH, Xu FX, Yan YH, Ding CF. A Ti/Nb-functionalized COF material based on IMAC strategy for efficient separation of phosphopeptides and phosphorylated exosomes. Anal Bioanal Chem. 2022;414:7885–95. https://doi.org/10.1007/s00216-022-04323-w.
Wang ZZ, Wei BB, Chen Z, Zhu HW, Shen GP, Feng JH. 1H nuclear magnetic resonance-based investigation of uremia by metabolomic analysis. Chin J Anal Chem. 2018;46:1415–1423. https://doi.org/10.11895/j.issn.0253.3820.181286.
Pedersen TX, McCormick SP, Tsimikas S, Bro S, Nielsen LB. Lipoprotein (a) accelerates atherosclerosis in uremic mice. J Lipid Res. 2010;51(10):2967–75. https://doi.org/10.1194/jlr.M006742.
Lee DY, Park SK, Yorgin PD, Cohen P, Oh Y, Rosenfeld RG. Alteration in insulin-like growth factor-binding proteins (IGFBPs) and IGFBP-3 protease activity in serum and urine from acute and chronic renal failure. J Clin Endocr Metab. 1994;79(5):1376–82. https://doi.org/10.1210/jc.79.5.1376.
Peerce BE, Clarke RD. Renal cytoplasmic proteasome proteinase activities are altered in chronic renal failure. Arch Biochem Biophys. 2005;444(2):84–91. https://doi.org/10.1016/j.abb.2005.09.012.
Stachowski J, Pollok M, Burrichter H, Spithaler C, Baldamus CA. Signalling via the TCR/CD3 antigen receptor complex in uremia is limited by the receptors number. Nephron. 1993;64(3):369–75. https://doi.org/10.1159/000187356.
Funding
This research is financed by the Natural Science Foundation of Zhejiang Province (LY22B050008).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
Samples of human serum were obtained from the Affiliated Hospital of Ningbo University with the approval of its Ethics Committee (KS20227002).
Consent to participate
The ethically approved human serum and saliva used in this study were collected with the consent of the volunteers. This study was approved by the experimental ethics committee of Ningbo University and its affiliated hospital.
Consent for publication
All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Hua, S., Feng, Q. et al. A novel hydrophilic polymer-coated magnetic nanomaterial based on the HILIC strategy for fast separation of glycopeptides and glycosylated exosomes. Anal Bioanal Chem 415, 5755–5767 (2023). https://doi.org/10.1007/s00216-023-04857-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04857-7