Abstract
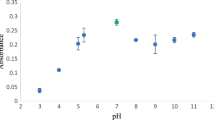
A new hybrid bionanomaterial composed of graphene oxide (GO) and Spirulina maxima (SM) algae was synthesized and applied to develop a preconcentration method based on the dispersive micro-solid phase extraction (D-μ-SPE) technique for the determination of Pb in water and infant beverages. In this work, Pb(II) was extracted with 3 mg of the hybrid bionanomaterial (GO@SM) followed by a back-extraction step using 500 µL of 0.6 mol L−1 HCl. Then, a 1.5 × 10−3 mol L−1 dithizone solution was added to the sample containing the analyte to form a purplish red-colored complex for its detection by UV–Vis spectrophotometry at 553 nm. An extraction efficiency of 98% was obtained after optimization of experimental variables such as GO@SM mass, pH, sample volume, type, and time of agitation. A detection limit of 1 μg L−1 and a relative standard deviation of 3.5% (at 5 μg L−1 Pb(II), n = 10) were achieved. The calibration linear range was obtained between 3.3 and 95 µg L−1 Pb(II). The proposed method was successfully applied for the preconcentration and determination of Pb(II) in infant beverages. Finally, the greenness degree of the D-µ-SPE method was evaluated using the Analytical GREEnness calculator (AGREE), obtaining a score of 0.62.
Graphical abstract




Similar content being viewed by others
Abbreviations
- AGREE:
-
Analytical GREEnness calculator
- ATR-FTIR:
-
Fourier transform infrared spectroscopy with attenuated total reflectance
- CRM:
-
Certified reference material
- D-µ-SPE:
-
Dispersive micro-solid phase extraction
- DTZ:
-
Dithizone
- EF:
-
Enhancement factor
- ETAAS:
-
Electrothermal atomic absorption spectrometry
- FAO:
-
The Food and Agriculture Organization of the United Nations
- GO:
-
Graphene oxide
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- LOD:
-
Limit of detection
- RSD:
-
Relative standard deviation
- SEM:
-
Scanning electron microscopy
- SM:
-
Spirulina maxima
- UV-Vis:
-
Ultraviolet-visible spectroscopy
- WHO:
-
World Health Organization
References
Rahman Z, Singh VP. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess. 2019;191(7):1–21. https://doi.org/10.1007/s10661-019-7528-7.
Rubio C, Gutiérrez AJ, Izquierdo REM, Revert C, Lozano G, Hardisson A. El plomo como contaminante alimentario. Rev Toxicol. 2004;21(2–3):72–80.
World Health Organization (WHO). Guidelines for drinking-water quality. Switzerland: WHO Library Cataloguing-in-Publication Data (4th Ed); 2004. 631 p.
Khan WA, Arain MB, Soylak M. Nanomaterials-based solid phase extraction and solid phase microextraction for heavy metals food toxicity. Food Chem Toxicol. 2020;145:111704. https://doi.org/10.1016/j.fct.2020.
Penner MH. Ultraviolet, visible, and fluorescence spectroscopy. Food Anal. 2017:89–106. https://doi.org/10.1007/978-3-319-45776-5_7.
Yilmaz E, Soylak M. Type of new generation separation and preconcentration methods. New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species: Elsevier; 2020. p. 75–148. https://doi.org/10.1016/B978-0-12-818569-8.00003-6.
Escudero LB, Quintas PY, Wuilloud RG, Dotto GL. Biosorption of metals and metalloids. In: Lichtfouse Ca, editor. Green Adsorbents for Pollutant Removal. 19: Springer Cham; 2018. p. 35–86. https://doi.org/10.1007/978-3-319-92162-4_2.
Ozdemir S, Kilinc E, Celik KS, Okumus V, Soylak M. Simultaneous preconcentrations of Co2+, Cr6+, Hg2+ and Pb2+ ions by Bacillus altitudinis immobilized nanodiamond prior to their determinations in food samples by ICP-OES. Food Chem. 2017;215:447–53. https://doi.org/10.1016/j.foodchem.2016.07.055.
Wang J, Liu M, Zhang L, Zhang T, Yue T, Li Z, et al. Biomass reinforced graphene oxide solid/liquid phase membrane extraction for the measurement of Pb (II) in food samples. Food Chem. 2018;269:9–15. https://doi.org/10.1016/j.foodchem.2018.06.137.
Moallaei H, Bouchara J-P, Rad A, Singh P, Raizada P, Tran HN, et al. Application of Fusarium sp. immobilized on multi-walled carbon nanotubes for solid-phase extraction and trace analysis of heavy metal cations. Food Chem. 2020;322:126757. https://doi.org/10.1016/j.foodchem.2020.
Suo L, Zhao J, Dong X, Gao X, Li X, Xu J, et al. Functionalization of a SiO 2-coated magnetic graphene oxide composite with polyaniline–polypyrrole for magnetic solid phase extraction of ultra-trace Cr (III) and Pb (II) in water and food samples using a Box-Behnken design. New J Chem. 2019;43(30):12126–36. https://doi.org/10.1039/C9NJ02038A.
Baytak S, Türker AR. The use of Agrobacterium tumefacients immobilized on Amberlite XAD-4 as a new biosorbent for the column preconcentration of iron (III), cobalt (II), manganese (II) and chromium (III). Talanta. 2005;65(4):938–45. https://doi.org/10.1016/j.talanta.2004.08.021.
Balaji S, Kalaivani T, Rajasekaran C, Shalini M, Siva R, Singh RK, et al. Arthrospira (Spirulina) species as bioadsorbents for lead, chromium, and cadmium: a comparative study. CLEAN–Soil, Air, Water. 2014;42(12):1790–7. https://doi.org/10.1002/clen.201300478.
Escudero LB, Maniero MA, Agostini E, Smichowski P. Biological substrates: green alternatives in trace elemental preconcentration and speciation analysis. TrAC. 2016;80:531–46. https://doi.org/10.1016/j.trac.2016.04.002.
Roto R, Mellisani B, Kuncaka A, Mudasir M, Suratman A. Colorimetric sensing of Pb2+ ion by using ag nanoparticles in the presence of dithizone. Chemosensors. 2019;7(3):28. https://doi.org/10.3390/chemosensors7030028.
Sotolongo AC, Martinis EM, Wuilloud RG. An easily prepared graphene oxide–ionic liquid hybrid nanomaterial for micro-solid phase extraction and preconcentration of Hg in water samples. Anal Methods. 2018;10(3):338–46. https://doi.org/10.1039/C7AY02201H.
Muniyalakshmi M, Sethuraman K, Silambarasan D. Synthesis and characterization of graphene oxide nanosheets. Mater Today: Proc. 2020;21:408–10. https://doi.org/10.1016/j.matpr.2019.06.375.
Lebron YAR, Moreira VR, Santos LVS. Studies on dye biosorption enhancement by chemically modified Fucus vesiculosus, Spirulina maxima and Chlorella pyrenoidosa algae. J Clean Prod. 2019;240:118197. https://doi.org/10.1016/j.jclepro.2019.
Zhang S, Wang H, Liu J, Bao C. Measuring the specific surface area of monolayer graphene oxide in water. Mater Lett. 2020;261:127098. https://doi.org/10.1016/j.matlet.2019.
Sanchez C, Shea KJ, Kitagawa S. Recent progress in hybrid materials science. Chem Soc Rev. 2011;40(2):471–2. https://doi.org/10.3390/separations6040056.
Zhang Q, Xue C, Owens G, Chen Z. Preparation of bionanomaterial based on green reduced graphene immobilized Ochrobactrum sp. FJ1: optimization, characterization and its application. Sep Purif Technol. 2023:123144. https://doi.org/10.1016/j.seppur.2023.
Wahid MH, Eroglu E, Chen X, Smith SM, Raston CL. Entrapment of Chlorella vulgaris cells within graphene oxide layers. RSC Adv. 2013;3(22):8180–3. https://doi.org/10.1039/c3ra40605a.
Wahid MH, Eroglu E, Chen X, Smith SM, Raston CL. Functional multi-layer graphene–algae hybrid material formed using vortex fluidics. Green Chem. 2013;15(3):650–5. https://doi.org/10.1039/c2gc36892g.
Muthoosamy K, Manickam S. State of the art and recent advances in the ultrasound-assisted synthesis, exfoliation and functionalization of graphene derivatives. Ultrason Sonochem. 2017;39:478–93. https://doi.org/10.1016/j.ultsonch.2017.05.019.
Romberg B, Müller H. Photometric screening-test for heavy metals under flow injection conditions using extractive determination with dithizone. Anal Chim Acta. 1997;353(2–3):165–72. https://doi.org/10.1016/S0003-2670(97)87774-4.
Syahrir M, Wijaya M, Irmayani R, editors. The comparison of optimum conditions for lead (Pb) analysis method using DITHIZONE and EDTA complex in seaweeds (Eucheuma spinosum). J Phys Conf Ser. 2021;752:12059. https://iopscience.iop.org/article/10.1088/1742-6596/1752/1/012059.
Maratta A, Vazquez S, Lopez A, Augusto M, Pacheco PH. Lead preconcentration by solid phase extraction using oxidized carbon xerogel and spectrophotometric determination with dithizone. Microchem J. 2016;128:166–71. https://doi.org/10.1016/j.microc.2016.04.017.
Javaid A, Bajwa R, Shafique U, Anwar J. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass Bioenerg. 2011;35(5):1675–82. https://doi.org/10.1016/j.biombioe.2010.12.035.
Mousavi HZ, Aibaghi-Esfahani B, Arjmandi A. Solid phase extraction of lead (II) by sorption on grinded eucalyptus stem and determination with flame atomic absorption spectrometry. J Chin Chem Soc. 2009;56(5):974–80. https://doi.org/10.1002/jccs.200900142.
Al-Dhabi NA, Arasu MV. Biosorption of hazardous waste from the municipal wastewater by marine algal biomass. Environ Res. 2022;204. https://doi.org/10.1016/j.jhazmat.2021.126336.
Ahmad A, Bhat AH, Buang A. Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: kinetic and equilibrium modeling. J Clean Prod. 2018;171:1361–75. https://doi.org/10.1016/j.jclepro.2017.09.252.
Ozdemir S, Kilinc E, Oner ET. Preconcentrations and determinations of copper, nickel and lead in baby food samples employing Coprinus silvaticus immobilized multi-walled carbon nanotube as solid phase sorbent. Food Chem. 2019;276:174–9. https://doi.org/10.1016/j.foodchem.2018.07.123.
Cui L, Wang Y, Gao L, Hu L, Yan L, Wei Q, et al. EDTA functionalized magnetic graphene oxide for removal of Pb (II), Hg (II) and Cu (II) in water treatment: adsorption mechanism and separation property. J Chem Eng. 2015;281:1–10. https://doi.org/10.1016/j.cej.2015.06.043.
Arribas Jimeno S, Hernández Méndez J, Lucena Conde F, Burriel Martí, F. Química analítica cualitativa. Madrid, España: Paraninfo (Ed), S.A; 2002. 1072 p.
Mohubedu RP, Diagboya PN, Abasi CY, Dikio ED, Mtunzi F. Magnetic valorization of biomass and biochar of a typical plant nuisance for toxic metals contaminated water treatment. J Clean Prod. 2019;209:1016–24. https://doi.org/10.1016/j.jclepro.2018.10.215.
de Paiva EL, Morgano MA, Arisseto-Bragotto AP. Occurrence and determination of inorganic contaminants in baby food and infant formula. Curr Opin Food Sci. 2019;30:60–6. https://doi.org/10.1016/j.cofs.2019.05.006.
Miller J, Miller JC. Statistics and chemometrics for analytical chemistry. England: Pearson Education Limited (5th Ed); 2005. 268 p.
Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE—Analytical GREEnness metric approach and software. Anal Chem. 2020;92(14):10076–82. https://doi.org/10.1021/acs.analchem.0c01887.
Gardener H, Bowen J, Callan SP. Lead and cadmium contamination in a large sample of United States infant formulas and baby foods. Sci Total Environ. 2019;651:822–7. https://doi.org/10.1016/j.scitotenv.2018.09.026.
Barbosa VMP, Barbosa AF, Bettini J, Luccas PO, Figueiredo EC. Direct extraction of lead (II) from untreated human blood serum using restricted access carbon nanotubes and its determination by atomic absorption spectrometry. Talanta. 2016;147:478–84. https://doi.org/10.1016/j.talanta.2015.10.023.
Khan M, Yilmaz E, Soylak M. Vortex assisted magnetic solid phase extraction of lead (II) and cobalt (II) on silica coated magnetic multiwalled carbon nanotubes impregnated with 1-(2-pyridylazo)-2-naphthol. J Mol Liq. 2016;224:639–47. https://doi.org/10.1016/j.molliq.2016.10.023.
Lotfi Z, Zavvar Mousavi H, Sajjadi SM. Nitrogen doped nano porous graphene as a sorbent for separation and preconcentration trace amounts of Pb, Cd and Cr by ultrasonic assisted in-syringe dispersive micro solid phase extraction. Appl Organomet Chem. 2018;32(3):e4162. https://doi.org/10.1002/aoc.4162.
Chaikhan P, Udnan Y, Ampiah-Bonney RJ, Chaiyasith WC. Magnetic dispersive solid phase extraction using recycled-graphite for GO-Fe3O4-dithizone composite combined with FAAS for determination of lead in environmental samples. Anal Sci. 2021:1015–21. https://doi.org/10.2116/analsci.20P383.
dos Santos PM, Tarley CRT, Corazza MZ. Dispersive solid-phase extraction using 3 mercaptopropyltrimethoxysilane functionalized magnetic MWCNT-based nanocomposite for selective and efficient preconcentration of Pb2+ with FAAS determination. J Braz Chem Soc. 2022;33:37–47. https://doi.org/10.21577/0103-5053.20210122.
Lari A, Esmaeili N, Ghafari H. Ionic liquid functionlized on multiwall carbon nanotubes for nickel and lead determination in human serum and urine samples by micro solid-phase extraction. Anal Methods Environ Chem J. 2021;4(02):72–85. https://doi.org/10.24200/amecj.v4.i02.144.
Funding
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIBAA 1208), Universidad Nacional de Cuyo (M015–T1: “Graphene oxide nanomaterials functionalized with amino acids for the adsorptive removal of toxic dyes from water and industrial effluents”), and Organization for the Prohibition of Chemical Weapons (OPCW Project: “Hybrid bio–nanomaterials: tools for the development of highly sensitive analytical methods applied to the determination of toxic elements in baby food”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ingrassia, E.B., Fiorentini, E.F., Wuilloud, R.G. et al. Novel bionanomaterial based on Spirulina maxima algae and graphene oxide for lead microextraction and determination in water and infant beverages. Anal Bioanal Chem 415, 5475–5486 (2023). https://doi.org/10.1007/s00216-023-04821-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04821-5