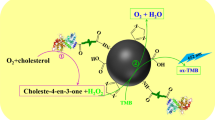
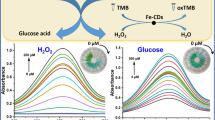
Abstract
Nanozyme, with enzyme-mimicking activity and excellent stability, has attracted extensive attention. However, some inherent disadvantages, including poor dispersion, low selectivity, and insufficient peroxidase-like activity, still limit its further development. Therefore, an innovative bioconjugation of a nanozyme and natural enzyme was conducted. In the presence of graphene oxide (GO), histidine magnetic nanoparticles (H-Fe3O4) were first synthesized by a solvothermal method. The GO-supported H-Fe3O4 (GO@H-Fe3O4) exhibited superior dispersity and biocompatibility because GO was the carrier and possessed outstanding peroxidase-like activity because of the introduction of histidine. Furthermore, the mechanism of the peroxidase-like activity of GO@H-Fe3O4 was the generation of •OH. Uric acid oxidase (UAO) was selected as the model natural enzyme and covalently linked to GO@H-Fe3O4 with hydrophilic poly(ethylene glycol) as a linker. UAO could specifically catalyze the oxidation of uric acid (UA) to generate H2O2, and subsequently, the newly produced H2O2 oxidized the colorless 3,3′,5,5′-tetramethylbenzidine (TMB) to blue ox-TMB under the catalysis of GO@H-Fe3O4. Based on the above cascade reaction, the GO@H-Fe3O4-linked UAO (GHFU) and GO@H-Fe3O4-linked ChOx (GHFC) were used for the detection of UA in serum samples and cholesterol (CS) in milk, respectively. The method based on GHFU exhibited a wide detection range (5–800 μM) and a low detection limit (1.5 μM) for UA, and the method based on GHFC exhibited a wide detection range (4–400 μM) and a low detection limit (1.13 μM) for CS. These results demonstrated that the proposed strategy had great potential in the field of clinical detection and food safety.
Graphical Abstract








Similar content being viewed by others
References
Liang J, Liang K. Multi-enzyme cascade reactions in metal-organic frameworks. Chem Rec. 2020;20(10):1100–16. https://doi.org/10.1002/tcr.202000067.
Wang D, Chai Y, Yuan Y, Yuan R. Lattice-like DNA tetrahedron nanostructure as scaffold to locate GOx and HRP enzymes for highly efficient enzyme cascade reaction. ACS Appl Mater Interfaces. 2020;12(2):2871–7. https://doi.org/10.1021/acsami.9b18702.
Becker M, Nikel P, Andexer JN, Lutz S, Rosenthal K. A Multi-enzyme cascade reaction for the production of 2′3′-cGAMP. Biomolecules. 2021;11(4):590. https://doi.org/10.3390/biom11040590.
Zhao J, Wang S, Lu S, Bao X, Sun J, Yang X. An enzyme cascade-triggered fluorogenic and chromogenic reaction applied in enzyme activity assay and immunoassay. Anal Chem. 2018;90(12):7754–60. https://doi.org/10.1021/acs.analchem.8b01845.
Yan Y, Qiao Z, Hai X, Song W, Bi S. Versatile electrochemical biosensor based on bi-enzyme cascade biocatalysis spatially regulated by DNA architecture. Biosens Bioelectron. 2021;174:112827. https://doi.org/10.1016/j.bios.2020.112827.
Wang Y, Jia G, Cui X, Zhao X, Zhang Q, Gu L, Zheng L, Li LH, Wu Q, Singh DJ, Matsumura D, Tsuji T, Cui Y-T, Zhao J, Zheng W. Coordination number regulation of molybdenum single-atom nanozyme peroxidase-like specificity. Chem. 2021;7(2):436–49. https://doi.org/10.1016/j.chempr.2020.10.023.
Li Y, Li S, Bao M, Zhang L, Carraro C, Maboudian R, Liu A, Wei W, Zhang Y, Liu S. Pd nanoclusters confined in ZIF-8 matrixes for fluorescent detection of glucose and cholesterol. ACS Applied Nano Materials. 2021;4(9):9132–42. https://doi.org/10.1021/acsanm.1c01712.
Xu Q, Yuan H, Dong X, Zhang Y, Asif M, Dong Z, He W, Ren J, Sun Y, Xiao F. Dual nanoenzyme modified microelectrode based on carbon fiber coated with AuPd alloy nanoparticles decorated graphene quantum dots assembly for electrochemical detection in clinic cancer samples. Biosens Bioelectron. 2018;107:153–62. https://doi.org/10.1016/j.bios.2018.02.026.
Dehvari K, Chiu SH, Lin JS, Girma WM, Ling YC, Chang JY. Heteroatom doped carbon dots with nanoenzyme like properties as theranostic platforms for free radical scavenging, imaging, and chemotherapy. Acta Biomater. 2020;114:343–57. https://doi.org/10.1016/j.actbio.2020.07.022.
Wu GW, He SB, Peng HP, Deng HH, Liu AL, Lin XH, Xia XH, Chen W. Citrate-capped platinum nanoparticle as a smart probe for ultrasensitive mercury sensing. Anal Chem. 2014;86(21):10955–60. https://doi.org/10.1021/ac503544w.
Fu Z, Zeng W, Cai S, Li H, Ding J, Wang C, Chen Y, Han N, Yang R. Porous Au@Pt nanoparticles with superior peroxidase-like activity for colorimetric detection of spike protein of SARS-CoV-2. J Colloid Interface Sci. 2021;604:113–21. https://doi.org/10.1016/j.jcis.2021.06.170.
Sun L, Ding Y, Jiang Y, Liu Q. Montmorillonite-loaded ceria nanocomposites with superior peroxidase-like activity for rapid colorimetric detection of H2O2. Sens Actuators, B Chem. 2017;239:848–56. https://doi.org/10.1016/j.snb.2016.08.094.
Sun Y, Shi F, Niu Y, Zhang Y, Xiong F. Fe3O4@OA@Poloxamer nanoparticles lower triglyceride in hepatocytes through liposuction effect and nano-enzyme effect. Colloids Surf B Biointerfaces. 2019;184:110528. https://doi.org/10.1016/j.colsurfb.2019.110528.
Xu J, Qing T, Jiang Z, Zhang P, Feng B. Graphene oxide-regulated low-background aptasensor for the “turn on” detection of tetracycline. Spectrochimica Acta Part A: Mol Biomol Spectroscopy. 2021;260:119898. https://doi.org/10.1016/j.saa.2021.119898.
Petrucci R, Chiarotto I, Mattiello L, Passeri D, Rossi M, Zollo G, Feroci AM. Graphene oxide: a smart (starting) material for natural methylxanthines adsorption and detection. Molecules. 2019;24(23):4247. https://doi.org/10.3390/molecules24234247.
Deng Y, Gao Q, Ma J, Wang C, Wei Y. Preparation of a boronate affinity material with ultrahigh binding capacity for cis-diols by grafting polymer brush from polydopamine-coated magnetized graphene oxide. Mikrochim Acta. 2018;185(3):189. https://doi.org/10.1007/s00604-018-2732-7.
Dey N, Bhattacharya S. Nanomolar level detection of uric acid in blood serum and pest-infested grain samples by an amphiphilic probe. Anal Chem. 2017;89(19):10376–83. https://doi.org/10.1021/acs.analchem.7b02344.
Yang Y, Song Y, Bo X, Min J, Pak OS, Zhu L, Wang M, Tu J, Kogan A, Zhang H, Hsiai TK, Li Z, Gao W. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat Biotechnol. 2020;38(2):217–24. https://doi.org/10.1038/s41587-019-0321-x.
Menotti A, Lanti M, Zanchetti A, Botta G, Laurenzi M, Terradura-Vagnarelli O. Mancini M The role of HDL cholesterol in metabolic syndrome predicting cardiovascular events. The Gubbio population study. Nutr Metab Cardiovasc Dis. 2011;21(5):315–22. https://doi.org/10.1016/j.numecd.2009.11.001.
Li X, Kong C, Chen Z. Colorimetric sensor arrays for antioxidant discrimination based on the inhibition of the oxidation reaction between 3,3′,5,5′-tetramethylbenzidine and hydrogen peroxides. ACS Appl Mater Interfaces. 2019;11(9):9504–9. https://doi.org/10.1021/acsami.8b18548.
Qu S, Li Z, Jia Q. Detection of purine metabolite uric acid with picolinic-acid-functionalized metal-organic frameworks. ACS Appl Mater Interfaces. 2019;11(37):34196–202. https://doi.org/10.1021/acsami.9b07442.
Batra N, Tomar M, Gupta V. ZnO-CuO composite matrix based reagentless biosensor for detection of total cholesterol. Biosens Bioelectron. 2015;67:263–71. https://doi.org/10.1016/j.bios.2014.08.029.
Jeon WY, Lee CJ, Sut TN, Kim HH, Choi YB. Pentacyanoammineferrate-based non-enzymatic electrochemical biosensing platform for selective uric acid measurement. Sensors (Basel). 2021;21(5):1574. https://doi.org/10.3390/s21051574.
Faruk Hossain M, Slaughter G. Flexible electrochemical uric acid and glucose biosensor. Bioelectrochemistry. 2021;141:107870. https://doi.org/10.1016/j.bioelechem.2021.107870.
Kim I, Kim YI, Lee SW, Jung HG, Lee G. Yoon DS Highly permselective uric acid detection using kidney cell membrane-functionalized enzymatic biosensors. Biosens Bioelectron. 2021;190:113411. https://doi.org/10.1016/j.bios.2021.113411.
Hassanzadeh J, Khataee A. Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta. 2018;178:992–1000. https://doi.org/10.1016/j.talanta.2017.08.107.
Fan K, Wang H, Xi J, Liu Q, Meng X, Duan D, Gao L, Yan X. Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem Commun (Camb). 2016;53(2):424–7. https://doi.org/10.1039/c6cc08542c.
Ma H, Li M, Yu T, Zhang H, Xiong M, Li F. Magnetic ZIF-8-based mimic multi-enzyme system as a colorimetric biosensor for detection of aryloxyphenoxypropionate herbicides. ACS Appl Mater Interfaces. 2021;13(37):44329–38. https://doi.org/10.1021/acsami.1c11815.
Duan R, Peng C, Sun L, Zhang LX, Bai CC, Dong LY. Wang XH Integrating boronate affinity controllable-oriented surface imprinting nylon wire and pH-triggered allochroic-graphene oxide for ultrasensitive detection of glycoprotein. Sensors and Actuators B: Chemical. 2021;330:129310. https://doi.org/10.1016/j.snb.2020.129310.
Wang Q, Zhang X, Huang L, Zhang Z, Dong S. GOx@ZIF-8(NiPd) nanoflower: an artificial enzyme system for tandem catalysis. Angew Chem Int Ed Engl. 2017;56(50):16082–5. https://doi.org/10.1002/anie.201710418.
Li W, Fan GC, Gao F, Cui Y, Wang W, Luo X. High-activity Fe3O4 nanozyme as signal amplifier: a simple, low-cost but efficient strategy for ultrasensitive photoelectrochemical immunoassay. Biosens Bioelectron. 2019;127:64–71. https://doi.org/10.1016/j.bios.2018.11.043.
Liu Q, Wan K, Shang Y, Wang ZG, Zhang Y, Dai L, Wang C, Wang H, Shi X, Liu D, Ding B. Cofactor-free oxidase-mimetic nanomaterials from self-assembled histidine-rich peptides. Nat Mater. 2021;20(3):395–402. https://doi.org/10.1038/s41563-020-00856-6.
Xu J, Yuan Y, Zhang R, Song Y, Sui T, Wang J, Wang C, Chen Y, Guan S, Wang L. A deuterohemin peptide protects a transgenic Caenorhabditis elegans model of Alzheimerʼs disease by inhibiting Abeta1-42 aggregation. Bioorg Chem. 2019;82:332–9. https://doi.org/10.1016/j.bioorg.2018.10.072.
Zhang C, Chen C, Zhao D, Kang G, Liu F, Yang F, Lu Y, Sun J. Multienzyme cascades based on highly efficient metal-nitrogen-carbon nanozymes for construction of versatile bioassays. Anal Chem. 2022;94(8):3485–93. https://doi.org/10.1021/acs.analchem.1c04018.
Wang Q, Wen X, Kong J. Recent progress on uric acid detection: a review. Crit Rev Anal Chem. 2020;50(4):359–75. https://doi.org/10.1080/10408347.2019.1637711.
Wang X, Yao Q, Tang X, Zhong H, Qiu P, Wang X. A highly selective and sensitive colorimetric detection of uric acid in human serum based on MoS2-catalyzed oxidation TMB. Anal Bioanal Chem. 2019;411(4):943–52. https://doi.org/10.1007/s00216-018-1524-6.
Cai N, Tan L, Li Y, Xia T, Hu T, Su X. Biosensing platform for the detection of uric acid based on graphene quantum dots and G-quadruplex/hemin DNAzyme. Anal Chim Acta. 2017;965:96–102. https://doi.org/10.1016/j.aca.2017.01.067.
Omar MN, Salleh AB, Lim HN, Ahmad TA. Electrochemical detection of uric acid via uricase-immobilized graphene oxide. Anal Biochem. 2016;509:135–41. https://doi.org/10.1016/j.ab.2016.06.030.
Pan Y, Yang Y, Pang Y, Shi Y, Long Y, Zheng H. Enhancing the peroxidase-like activity of ficin via heme binding and colorimetric detection for uric acid. Talanta. 2018;185:433–8. https://doi.org/10.1016/j.talanta.2018.04.005.
Wang X, Li F, Cai Z, Liu K, Li J, Zhang B, He J. Sensitive colorimetric assay for uric acid and glucose detection based on multilayer-modified paper with smartphone as signal readout. Anal Bioanal Chem. 2018;410(10):2647–55. https://doi.org/10.1007/s00216-018-0939-4.
Wang XY, Zhu GB, Cao WD, Liu ZJ, Pan CG, Hu WJ, Zhao WY, Sun JF. A novel ratiometric fluorescent probe for the detection of uric acid in human blood based on H2O2-mediated fluorescence quenching of gold/silver nanoclusters. Talanta. 2019;191:46–53. https://doi.org/10.1016/j.talanta.2018.08.015.
Nishan U, Ullah W, Muhammad N, Asad M, Afridi S, Khan M, Shah M, Khan N, Rahim A. Development of a nonenzymatic colorimetric sensor for the detection of uric acid based on ionic liquid-mediated nickel nanostructures. ACS Omega. 2022;7(30):26983–91. https://doi.org/10.1021/acsomega.2c04070.
Liu M, He Y, Zhou J, Ge Y, Zhou J, Song G. A “naked-eye” colorimetric and ratiometric fluorescence probe for uric acid based on Ti3C2 MXene quantum dots. Anal Chim Acta. 2020;1103:134–42. https://doi.org/10.1016/j.aca.2019.12.069.
Dewangan L, Korram J, Karbhal I, Nagwanshi R, Jena VK, Satnami ML. A colorimetric nanoprobe based on enzyme-immobilized silver nanoparticles for the efficient detection of cholesterol. RSC Adv. 2019;9(72):42085–95. https://doi.org/10.1039/c9ra08328f.
Chung M, Jang YJ, Kim MI. Convenient colorimetric detection of cholesterol using multi-enzyme co-incorporated organic-inorganic hybrid nanoflowers. J Nanosci Nanotechnol. 2018;18(9):6555–61. https://doi.org/10.1166/jnn.2018.15697.
Hong C, Zhang X, Wu C, Chen Q, Yang H, Yang D, Huang Z, Cai R, Tan W. On-site colorimetric detection of cholesterol based on polypyrrole nanoparticles. ACS Appl Mater Interfaces. 2020;12(49):54426–32. https://doi.org/10.1021/acsami.0c15900.
Wu Q, He L, Jiang ZW, Li Y, Cao ZM, Huang CZ, Li YF. CuO nanoparticles derived from metal-organic gel with excellent electrocatalytic and peroxidase-mimicking activities for glucose and cholesterol detection. Biosens Bioelectron. 2019;145:111704. https://doi.org/10.1016/j.bios.2019.111704.
Zhao L, Wu Z, Liu G, Lu H, Gao Y, Liu F, Wang C, Cui J, Lu G. High-activity Mo, S co-doped carbon quantum dot nanozyme-based cascade colorimetric biosensor for sensitive detection of cholesterol. J Mater Chem B. 2019;7(44):7042–51. https://doi.org/10.1039/c9tb01731c.
Chang HC, Ho JA. Gold nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal Chem. 2015;87(20):10362–7. https://doi.org/10.1021/acs.analchem.5b02452.
Zhang Y, Wang Y, Zhou Q, Chen X, Jiao W, Li G, Peng M, Liu X, He Y, Fan H. Precise regulation of enzyme-nanozyme cascade reaction kinetics by magnetic actuation toward efficient tumor therapy. ACS Appl Mater Interfaces. 2021;13:52395–405. https://doi.org/10.1021/acsami.1c15717.
Dong H, Du W, Dong J, Che R, Kong F, Cheng W, Ma M, Gu N, Zhang Y. Depletable peroxidase-like activity of Fe3O4 nanozymes accompanied with separate migration of electrons and iron ions. Nat Commun. 2022;13(1):5365. https://doi.org/10.1038/s41467-022-33098-y.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21605114) and Tianjin Natural Science Foundation (Grant No. 17JCQNJC13300).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
The study (bc2022278) was approved by the institutional ethics committee of Tianjin Medical University Cancer Institute and Hospital (Tianjin, China), and carried out in according with the ethical guidelines of the Declaration of Helsinki for experiments involving humans. All healthy volunteers from our laboratory received a detailed description of the study and provided written informed consent to participate in the study before providing their serum samples.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lang, JY., Zhao, JM., Ren, MJ. et al. Bioconjugation of nanozyme and natural enzyme to enable a one-step cascade reaction for the detection of metabolites. Anal Bioanal Chem 415, 3385–3398 (2023). https://doi.org/10.1007/s00216-023-04720-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04720-9