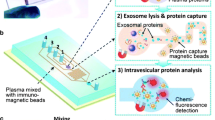
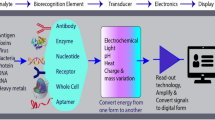
Abstract
The discovery of new molecular biomarkers of cancer during the last decades and the development of new diagnostic devices exploiting those have significantly contributed to the clinical analysis of cancer and to improve the outcomes. Among those, liquid biopsy sensors exploiting aptamers for the detection of cancer biomarkers in body fluids are useful and accurate tools for a fast and inexpensive non-invasive screening of population. The incorporation of aptamers in electrochemical sandwich biosensors using enzyme labels, a so-called ELASA, has demonstrated its utility to improve the detection schemes. In this review, we overview the existing ELASA assays for numerous cancer biomarkers as alternatives to the traditional ELISA and discuss their possibilities to reach the market, currently dominated by optical immunoassays.
Graphical abstract








Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. https://doi.org/10.3322/CAAC.21708.
Whitaker K. Earlier diagnosis: the importance of cancer symptoms. Lancet Oncol. 2020;21:6–8. https://doi.org/10.1016/S1470-2045(19)30687-4.
Definition of biomarker - NCI Dictionary of Cancer Terms - NCI. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomarker. Accessed 2 Sep 2022.
Duffy MJ. Tumor markers in clinical practice: a review focusing on common solid cancers. Med Princ Pract. 2013;22:4–11. https://doi.org/10.1159/000338393.
Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, Esener S, Fitzgerald RC, Gambhir SS, Kuhn P, Rebbeck TR, Balasubramanian S. Early detection of cancer. Science. 2022;375:eaay9040. https://doi.org/10.1126/science.aay9040.
Alix-Panabier̀es C, Pantel K,. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–8. https://doi.org/10.1373/CLINCHEM.2012.194258.
Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, El-Rifai W, Bedognetti D, Batra SK, Haris M, Bhat AA, Macha MA. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. 2021. https://doi.org/10.1186/s12943-022-01543-7.
Dragani TA, Matarese V, Colombo F. Biomarkers for early cancer diagnosis: prospects for success through the lens of tumor genetics. BioEssays. 2020;42:1–6. https://doi.org/10.1002/bies.201900122.
Ramanathan LV, Fleisher M, Duffy MJ. Cancer biomarkers : clinical aspects and laboratory determination. 1st ed. Elsevier; 2022.
Dunn MR, Jimenez RM. Chaput JC (2017) Analysis of aptamer discovery and technology. Nat Rev Chem. 2017;110(1):1–16. https://doi.org/10.1038/s41570-017-0076.
Cho EJ, Lee JW, Ellington AD. Applications of aptamers as sensors. Annu. Rev. Anal. Chem. 2009;2:241–264. https://doi.org/10.1146/annurev.anchem.1.031207.112851.
Hristova VA, Chan DW. Cancer biomarker discovery and translation: proteomics and beyond. 2018;6:93–103. https://doi.org/10.1080/14789450.2019.1559062.
Johnsen KB, Gudbergsson JM, Andresen TL, Simonsen JB. What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim Biophys Acta Rev Cancer. 2019;1871:109–16. https://doi.org/10.1016/J.BBCAN.2018.11.006.
Ludwig JA. Weinstein JN (2005) Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;511(5):845–56. https://doi.org/10.1038/nrc1739.
Malecka K, Mikuła E, Ferapontova EE. Design strategies for electrochemical aptasensors for cancer diagnostic devices. Sensors. 2021;21:736. https://doi.org/10.3390/S21030736.
Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8(4):268–278. https://doi.org/10.1038/nrc2351.
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti–HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. https://doi.org/10.1634/THEONCOLOGIST.2008-0230.
Campos-da-Paz M, Dórea JG, Galdino AS, Lacava ZGM, de Fatima Menezes Almeida Santos M. Carcinoembryonic antigen (CEA) and hepatic metastasis in colorectal cancer: update on biomarker for clinical and biotechnological approaches. Recent Pat Biotechnol. 2018;12:269–279. https://doi.org/10.2174/1872208312666180731104244.
Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. https://doi.org/10.1016/J.CCR.2006.10.002.
Skarmoutsos A, Skarmoutsos I, Katafigiotis I, Tataki E, Giagini A, Alamanis C, Anastasiou I, Angelou A, Duvdevani M, Sitaras N, Constantinides C. Detecting novel urine biomarkers for the early diagnosis of prostate cancer: platelet derived growth factor-BB as a possible new target. Curr Urol. 2018;12:13–9. https://doi.org/10.1159/000447225.
Moniaux N, Chakraborty S, Yalniz M, Gonzalez J, Shostrom VK, Standop J, Lele SM, Ouellette M, Pour PM, Sasson AR, Brand RE, Hollingsworth MA, Jain M. Batra SK (2008) Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008;989(98):1540–7. https://doi.org/10.1038/sj.bjc.6604329.
Florea A, Ravalli A, Cristea C, Săndulescu R, Marrazza G. An optimized bioassay for mucin1 detection in serum samples. Electroanalysis. 2015;27:1594–601. https://doi.org/10.1002/elan.201400689.
Wang X, Tian L, Lu J, Ng IOL. Exosomes and cancer - diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022;11:54. https://doi.org/10.1038/s41389-022-00431-5.
Chinen LTD, Abdallah EA, Braun AC, Flores B de CT de CP, Corassa M, Sanches SM, Fanelli MF. Circulating tumor cells as cancer biomarkers in the clinic. Adv Exp Med Biol. 2017;994:1–41. https://doi.org/10.1007/978-3-319-55947-6_1.
Saylan Y, Özgür E, Denizli A. Recent advances of medical biosensors for clinical applications. Med Devices Sensors 2021;4. https://doi.org/10.1002/MDS3.10129.
Hornbeck P. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 1992;1:2.1.1–2.1.22. https://doi.org/10.1002/0471142735.IM0201S01.
Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem. 2005;51:2415–8. https://doi.org/10.1373/CLINCHEM.2005.051532.
Kudłak B, Wieczerzak M. Aptamer based tools for environmental and therapeutic monitoring: a review of developments, applications, future perspectives. Crit Rev Environ Sci Technol. 2019;50:816–67. https://doi.org/10.1080/10643389.2019.1634457.
Koyappayil A, Lee MH. Ultrasensitive materials for electrochemical biosensor labels. Sensors 2021. 2020;21:89 21:89. https://doi.org/10.3390/S21010089.
Hempen C, Karst U. Labeling strategies for bioassays. Anal Bioanal Chem. 2006;384:572–83. https://doi.org/10.1007/S00216-005-3392-0.
Svigelj R, Zuliani I, Grazioli C, Dossi N, Toniolo R. An effective label-free electrochemical aptasensor based on gold nanoparticles for gluten detection. Nanomater (Basel, Switzerland) 2022;12. https://doi.org/10.3390/NANO12060987.
Berglund GI, Carlsson GH, Smith AT, Szöke H, Henriksen A, Hajdu J. The catalytic pathway of horseradish peroxidase at high resolution. Nature. 2002;417:463–8. https://doi.org/10.1038/417463a.
Wohlfahrt G, Witt S, Hendle J, Schomburg D, Kalisz HM, Hecht HJ. 1.8 and 1.9 Å resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Cryst. 1999;D55:969-977. https://doi.org/10.1107/S0907444999003431.
Murphy JE, Tibbitts TT, Kantrowitz ER. Mutations at positions 153 and 328 in Escherichia coli alkaline phosphatase provide insight towards the structure and function of mammalian and yeast alkaline phosphatases. J Mol Biol. 1995;253:604–17. https://doi.org/10.1006/JMBI.1995.0576.
Parsiegla G, Belaïch A, Belaïch JP, Haser R. Crystal structure of the cellulase Ce19M enlightens structure/function relationships of the variable catalytic modules in glycoside hydrolases. Biochemistry. 2002;41:11134–42. https://doi.org/10.1021/BI025816M/.
de Oliveira FK, Santos LO, Buffon JG. Mechanism of action, sources, and application of peroxidases. Food Res Int. 2021;143:110266. https://doi.org/10.1016/J.FOODRES.2021.110266.
Chen D, Wang D, Hu X, Long G, Zhang Y, Zhou L. A DNA nanostructured biosensor for electrochemical analysis of HER2 using bioconjugate of GNR@Pd SSs—Apt—HRP. Sensors Actuators B Chem. 2019;296. https://doi.org/10.1016/j.snb.2019.126650.
Zhao J, Zhang Y, Li H, Wen Y, Fan X, Lin F, Tan L, Yao S. Ultrasensitive electrochemical aptasensor for thrombin based on the amplification of aptamer-AuNPs-HRP conjugates. Biosens Bioelectron. 2011;26:2297–303. https://doi.org/10.1016/j.bios.2010.09.056.
Ou D, Sun D, Lin X, Liang Z, Zhong Y, Chen Z. A dual-aptamer-based biosensor for specific detection of breast cancer biomarker HER2 via flower-like nanozymes and DNA nanostructures. J Mater Chem B. 2019;7:3661–9. https://doi.org/10.1039/c9tb00472f.
Díaz-Fernández A, Miranda-Castro R, de-los-Santos-Álvarez N, Rodríguez EF, Lobo-Castañón MJ. Focusing aptamer selection on the glycan structure of prostate-specific antigen: toward more specific detection of prostate cancer. Biosens Bioelectron. 2019. https://doi.org/10.1016/j.bios.2018.12.040.
Wang P, Wan Y, Deng S, Yang S, Su Y, Fan C, Aldalbahi A, Zuo X. Aptamer-initiated on-particle template-independent enzymatic polymerization (aptamer-OTEP) for electrochemical analysis of tumor biomarkers. Biosens Bioelectron. 2016;86:536–41. https://doi.org/10.1016/j.bios.2016.07.025.
Zhang W, Tian Z, Yang S, Rich J, Zhao S, Klingeborn M, Huang PH, Li Z, Stout A, Murphy Q, Patz E, Zhang S, Liu G, Huang TJ. Electrochemical micro-aptasensors for exosome detection based on hybridization chain reaction amplification. Microsystems Nanoeng. 2021;7. https://doi.org/10.1038/s41378-021-00293-8.
Juska VB, Pemble ME. A critical review of electrochemical glucose sensing: evolution of biosensor platforms based on advanced nanosystems. Sensors. 2020;20(21):6013 https://doi.org/10.3390/s20216013.
Bauer JA, Zámocká M, Majtán J, Bauerová-Hlinková V. Glucose oxidase, an enzyme “Ferrari”: its structure, function, production and properties in the light of various industrial and biotechnological applications. Biomol 2022;12:472. https://doi.org/10.3390/BIOM12030472.
Zhang JJ, Cao JT, Shi GF, Huang KJ, Liu YM, Ren SW. A luminol electrochemiluminescence aptasensor based on glucose oxidase modified gold nanoparticles for measurement of platelet-derived growth factor BB. Talanta. 2015;132:65–71. https://doi.org/10.1016/j.talanta.2014.08.058.
Bai L, Yuan R, Chai Y, Yuan Y, Wang Y, Xie S. Direct electrochemistry and electrocatalysis of a glucose oxidase-functionalized bioconjugate as a trace label for ultrasensitive detection of thrombin. Chem Commun. 2012;48:10972–4. https://doi.org/10.1039/c2cc35295h.
Yan Y, Qiao Z, Hai X, Song W, Bi S. Versatile electrochemical biosensor based on bi-enzyme cascade biocatalysis spatially regulated by DNA architecture. Biosens Bioelectron. 2021;174:112827. https://doi.org/10.1016/j.bios.2020.112827.
Nsabimana A, Lan Y, Du F, Wang C, Zhang W, Xu G. Alkaline phosphatase-based electrochemical sensors for health applications. Anal Methods. 2019;11:1996–2006. https://doi.org/10.1039/C8AY02793E.
Fanjul-Bolado P, Herná ndez-Santos D, Begoñ Gonzá lez-García M, Costa-García A. Alkaline phosphatase-catalyzed silver deposition for electrochemical detection. Biosens Bioelectron. 2000;15:5272–5277. https://doi.org/10.1021/ac070624o.
Aydoğdu Tığ G, Pekyardımcı Ş. An electrochemical sandwich-type aptasensor for determination of lipocalin-2 based on graphene oxide/polymer composite and gold nanoparticles. Talanta 2020;210:120666. https://doi.org/10.1016/j.talanta.2019.120666.
Wang Y, Yuan R, Chai Y, Yuan Y, Bai L. In situ enzymatic silver enhancement based on functionalized graphene oxide and layer-by-layer assembled gold nanoparticles for ultrasensitive detection of thrombin. Biosens Bioelectron. 2012;38:50–4. https://doi.org/10.1016/j.bios.2012.04.046.
Zhang K, Lv S, Zhou Q, Tang D. CoOOH nanosheets-coated g-C3N4/CuInS2 nanohybrids for photoelectrochemical biosensor of carcinoembryonic antigen coupling hybridization chain reaction with etching reaction. Sensors Actuators B Chem. 2020;307:127631. https://doi.org/10.1016/j.snb.2019.127631.
Han J, Zhuo Y, Chai Y, Yuan R, Xiang Y, Zhu Q, Liao N. Multi-labeled functionalized C60 nanohybrid as tracing tag for ultrasensitive electrochemical aptasensing. Biosens Bioelectron. 2013;46:74–9. https://doi.org/10.1016/j.bios.2013.02.020.
Wang YH, Xia H, Huang KJ, Wu X, Ma YY, Deng R, Lu YF, Han ZW. Ultrasensitive determination of thrombin by using an electrode modified with WSe 2 and gold nanoparticles, aptamer-thrombin-aptamer sandwiching, redox cycling, and signal enhancement by alkaline phosphatase. Microchim Acta. 2018;185:1–7. https://doi.org/10.1007/s00604-018-3028-7.
Degefa TH, Hwang S, Kwon D, Park JH, Kwak J. Aptamer-based electrochemical detection of protein using enzymatic silver deposition. Electrochim Acta. 2009;54:6788–91. https://doi.org/10.1016/j.electacta.2009.06.082.
Wang H, Liu Y, Liu C, Huang J, Yang P, Liu B. Microfluidic chip-based aptasensor for amplified electrochemical detection of human thrombin. Electrochem commun. 2010;12:258–61. https://doi.org/10.1016/j.elecom.2009.12.008.
Sosna M, Ferapontova EE. Electron transfer in binary hemin-modified alkanethiol self-assembled monolayers on gold: Hemin’s lateral and interfacial interactions. Langmuir. 2022;38:11180–90. https://doi.org/10.1021/ACS.LANGMUIR.2C01064.
Alsharabasy AM, Pandit A, Farràs P. Recent advances in the design and sensing applications of hemin/coordination polymer-based nanocomposites. Adv Mater. 2021;33. https://doi.org/10.1002/ADMA.202003883.
Li J, Wu H, Yan Y, Yuan T, Shu Y, Gao X, Zhang L, Li S, Ding S, Cheng W. Zippered G-quadruplex/hemin DNAzyme: exceptional catalyst for universal bioanalytical applications. Nucleic Acids Res. 2021;49:13031–44. https://doi.org/10.1093/NAR/GKAB1178.
Ferapontova EE. Direct peroxidase bioelectrocatalysis on a variety of electrode materials. Electroanalysis. 2004;16:1101–12. https://doi.org/10.1002/ELAN.200403003.
Sosna M, Leiva-Eriksson N, Bülow L, Ferapontova EE. Electrochemical characterization and bioelectrocatalytic H2O2 sensing of non-symbiotic hexa-coordinated sugar beet hemoglobins. ChemElectroChem. 2020;7:2114–22. https://doi.org/10.1002/CELC.202000358.
Fapyane D, Kékedy-Nagy L, Sakharov IY, Ferapontova EE. Electrochemistry and electrocatalysis of covalent hemin-G4 complexes on gold. J Electroanal Chem. 2018;812:174–9. https://doi.org/10.1016/J.JELECHEM.2017.11.053.
Zhang F, Liu Z, Han Y, Fan L, Guo Y. Sandwich electrochemical carcinoembryonic antigen aptasensor based on signal amplification of polydopamine functionalized graphene conjugate Pd-Pt nanodendrites. Bioelectrochemistry 2021;142:107947. https://doi.org/10.1016/j.bioelechem.2021.107947.
Ou D, Sun D, Liang Z, Chen B, Lin X, Chen Z. A novel cytosensor for capture, detection and release of breast cancer cells based on metal organic framework PCN-224 and DNA tetrahedron linked dual-aptamer. Sensors Actuators B Chem. 2019;285:398–404. https://doi.org/10.1016/j.snb.2019.01.079.
Qiao B, Guo Q, Jiang J, Qi Y, Zhang H, He B, Cai C, Shen J. An electrochemiluminescent aptasensor for amplified detection of exosomes from breast tumor cells (MCF-7 cells) based on G-quadruplex/hemin DNAzymes. Analyst. 2019;144:3668–75. https://doi.org/10.1039/c9an00181f.
Bagheri Hashkavayi A, Cha BS, Hwang SH, Kim J, Park KS. Highly sensitive electrochemical detection of circulating EpCAM-positive tumor cells using a dual signal amplification strategy. Sensors Actuators B Chem. 2021;343:130087. https://doi.org/10.1016/j.snb.2021.130087.
Zhuo Y, Ma M nan, Chai YQ, Zhao M, Yuan R. Amplified electrochemiluminescent aptasensor using mimicking bi-enzyme nanocomplexes as signal enhancement. Anal Chim Acta. 2014;809:47–53. https://doi.org/10.1016/j.aca.2013.09.060.
Jing P, Yi H, Xue S, Chai Y, Yuan R, Xu W. A sensitive electrochemical aptasensor based on palladium nanoparticles decorated graphene-molybdenum disulfide flower-like nanocomposites and enzymatic signal amplification. Anal Chim Acta. 2015;853:234–41. https://doi.org/10.1016/j.aca.2014.10.003.
Díaz-Fernández A, Ferapontova EE. Covalent Hemin/G4 complex-linked sandwich bioassay on magnetic beads for femtomolar HER-2/neu detection in human serum via direct electrocatalytic reduction of oxygen. Anal Chim Acta 2022;1219. https://doi.org/10.1016/j.aca.2022.340049.
Jayasekara S, Ratnayake R. Microbial cellulases: an overview and applications. In: Pascual AR, Martín MEE, editors Cellulose, IntechOpen, London. IntechOpen; 2019.
Malecka K, Pankratov D, Ferapontova EE. Femtomolar electroanalysis of a breast cancer biomarker HER-2/neu protein in human serum by the cellulase-linked sandwich assay on magnetic beads. Anal Chim Acta. 2019;1077:140–9. https://doi.org/10.1016/j.aca.2019.05.052.
Fapyane D, Ferapontova EE. Electrochemical assay for a total cellulase activity with improved sensitivity. Anal Chem. 2017;89:3959–65. https://doi.org/10.1021/ACS.ANALCHEM.6B04391.
Díaz-Fernández A, Vendelbo MH, Ferapontova EE. Electrochemical cellulase-linked ELASA for rapid liquid biopsy testing of serum HER-2/neu. ACS Measurement Science Au, 2023.
Díaz-Fernández A, Lorenzo-Gómez R, Miranda-Castro R, de-los-Santos-Álvarez N, Lobo-Castañón MJ. Electrochemical aptasensors for cancer diagnosis in biological fluids – a review. Anal Chim Acta 2020;1124:1–19. https://doi.org/10.1016/J.ACA.2020.04.022.
Horvath AR, Lord SJ, StJohn A, Sandberg S, Cobbaert CM, Lorenz S, Monaghan PJ, Verhagen-Kamerbeek WDJ, Ebert C, Bossuyt PMM. From biomarkers to medical tests: the changing landscape of test evaluation. Clin Chim Acta. 2014;427:49–57. https://doi.org/10.1016/J.CCA.2013.09.018.
Bruno JG. Predicting the uncertain future of aptamer-based diagnostics and therapeutics. Molecules. 2015;20:6866–87. https://doi.org/10.3390/molecules20046866.
Funding
The work was funded by the Novo Nordisk Foundation through the project “Validating Serum Tests for Human Epidermal growth factor Receptor-2 for Precise Diagnosis and Stratification of Breast and Gastro-Oesophagal Cancers”; the grant reference number NNF20OC0065428.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Young Investigators in (Bio-)Analytical Chemistry 2023 with guest editors Zhi-Yuan Gu, Beatriz Jurado-Sánchez, Thomas H. Linz, Leandro Wang Hantao, Nongnoot Wongkaew, and Peng Wu.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Díaz-Fernández, A., Ferapontova, E.E. Electrochemical ELASA: improving early cancer detection and monitoring. Anal Bioanal Chem 415, 3831–3846 (2023). https://doi.org/10.1007/s00216-023-04546-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04546-5