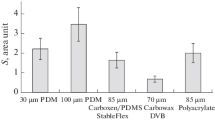
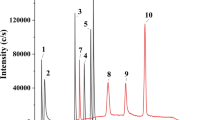
Abstract
This work describes the intricacies of the determination of the trimethylselenonium ion (TMSe) in human urine via high-performance liquid chromatography–hydride generation–atomic fluorescence spectrometry (HPLC-HG-AFS). By definition, this technique requires that the separated TMSe can be online converted into a volatile compound. Literature data for the determination of TMSe via the hydride generation technique are contradictory; i.e., some authors claim that direct formation of volatile compounds is possible under reduction with NaBH4, whereas others reported that a digestion step is mandatory prior to conversion. We studied and optimized the conditions for online conversion by varying the mobile phase composition (pyridine, phosphate, and acetate), testing different reaction coils, and optimizing the hydride generation conditions, although technically no hydride (H2Se) is formed but a dimethylselenide (DMSe). The optimized conditions were used for the analysis of 64 urine samples of 16 (unexposed) volunteers and the determination of low amounts of TMSe (LOD = 0.2 ng mL−1). Total (specific gravity–corrected) selenium concentrations in the urine samples ranged from 7.9 ± 0.7 to 29.7 ± 5.0 ng mL−1 for individual volunteers. Four volunteers were characterized as TMSe producers (hINMT genotype GA) and 12 were non-producers (hINMT genotype GG). Urine of TMSe producers contained 2.5 ± 1.7 ng mL−1 of TMSe, compared to 0.2 ± 0.2 ng mL−1 for non-producers.






Similar content being viewed by others
References
Ganther HE, Kraus RJ, Foster SJ. Trimethylselenonium ion, sulfur and sulfur amino acids. Meth Enzymol. 1987;143:195–201.
Suzuki KT, Kurasaki K, Okazaki N, Ogra Y. Selenosugar and trimethylselenonium among urinary Se metabolites: dose- and age-related changes. Toxicol Appl Pharmacol. 2005; 206 https://doi.org/10.1016/j.taap.2004.10.018
Jäger T, Drexler H, Göen T. Human metabolism and renal excretion of selenium compounds after oral ingestion of sodium selenite and selenized yeast dependent on the trimethylselenium ion (TMSe) status. Arch Toxicol. 2016. https://doi.org/10.1007/s00204-015-1548-z.
Juresa D, Blanusa A, Francesconi KA, Kienzl N, Kuehnelt D. Biological availability of selenosugars in rats. Chem Biol Interact. 2007. https://doi.org/10.1016/j.cbi.2007.04.009.
Moreno P, Quijano MA, Guttiérez AM, Pérez-Conde MC, Cámara C. Study of selenium species distribution in biological tissues by size exclusion and ion exchange chromatography inductively coupled plasma-mass spectrometry. Anal Chim Acta. 2004. https://doi.org/10.1016/j.aca.2004.02.029.
Kuehnelt D, Juresa D, Kienzl N, Francesconi KA. Marked individual variability in the levels of trimethylselenonium ion in human urine determined by HPLC/ICPMS and HPLC/vapor generation/ICPMS. Anal Bioanal Chem. 2006. https://doi.org/10.1007/s00216-006-0848-9.
Kuehnelt D, Engström K, Skröder H, Kokarnig S, Schlebusch C, Kippler M, Alhamdow A, Nermell B, Francesconi KA, Broberg K, Vahter M. Selenium metabolism to the trimethylselenonium ion (TMSe) varies markedly because of polymorphisms in the indolethylamine N-methyltransferase gene. Am J Clin Nutr. 2015. https://doi.org/10.3945/ajcn.115.114157.
Lajin B, Kuehnelt D, Jensen KB, Francesconi KA. Investigating the intra-individual variability in the human metabolic profile of urinary selenium. J Trace Elem Med Biol. 2016. https://doi.org/10.1016/j.jtemb.2016.06.008.
Maher W, Krikowa F, Foster S. Decomposition of six common selenium species found in animal tissues using microwave digestion with nitric acid and ICP-MS. Microchem J. 2016. https://doi.org/10.1016/j.microc.2015.11.009.
Li F, Goessler W, Irgolic KJ. Optimization of microwave digestion for determination of selenium in human urine by flow injection-hydride generation-atomic absorption spectrometry. Anal Commun. 1998;35:361–4.
Hildebrand J, Greiner A, Drexler H, Goen T. Determination of eleven small selenium species in human urine by chromatographic-coupled ICP-MS methods. J Trace Elem Med Biol. 2020. https://doi.org/10.1016/j.jtemb.2020.126519.
Delafiori AJ, Ring G, Furey A. Clinical applications of HPLC–ICP-MS element speciation: a review. Talanta. 2016. https://doi.org/10.1016/j.talanta.2016.02.035.
Gammelgaard B, Jons O. Determination of selenium in urine by inductively coupled plasma mass spectrometry: interferences and optimization. J Anal At Spectrom. 1999;14:867–74.
Blais JS, Huyghuesdespointes A, Momplaisir GM, Marshall WD. High-performance liquid-chromatography atomic-absorption spectrometry interface for the determination of selenoniocholine and trimethylselenonium cations - application to human urine. J Anal At Spectrom. 1991;6:225–32.
Chatterjee A, Shibata Y, Yoneda M, Banerjee R, Uchida M, Kon H, Morita M. Identification of volatile selenium compounds produced in the hydride generation system from organoselenium compounds. Anal Chem. 2001;73:3181–6.
D’Ulivo A, Lampugnani L, Sfetsios I, Zamboni R. Studies on total selenium determination in biological samples by hydride generation nondispersive atomic fluorescence spectrometry after hydrobromic acid bromine wet digestion. Spectrochim Acta Part B. 1993;48:387–96.
D’Ulivo A, Lampugnani L, Sfetsios I, Zamboni R, Forte C. Studies on the breakdown of organoselenium compounds in a hydrobromic acid bromine digestion system. Analyst. 1994;119:633–40.
Gómez MM, Gasparic T, Palacios MA, Camara C. Determination of five selenium compounds in urine by liquid chromatography with focused microwave assisted digestion and hydride generation-atomic absorption spectrometric detection. Anal Chim Acta. 1998;374:241–51.
Cobo-Fernández MG, Palacios MA, Chakraborti D, Quevauviller P, Cámara C. On line speciation of Se(VI) and Se(IV), and trimethylselenium by HPLC-microvawe oven-hydride generation-atomic absorption spectrometry. Fresenius J Anal Chem. 1995;351:438–42.
Zhang YQ, Frankenberg WT. Speciation of selenium in plant water extracts by ion exchange chromatography-hydride generation atomic absorption spectrometry. Sci Tot Environ. 2001. https://doi.org/10.1016/S0048-9697(00)00809-3.
Chatterjee A, Irgolic KJ. Behaviour of selenium compounds in FI-HG-AAS. Anal Commun. 1998;35:337–40.
Chatterjee A, Shibata Y. Determination of trimethylselenonium ion by flow injection hydride generation atomic absorption spectrometry. Anal Chim Acta. 1999;398:273–8.
Zhao QX, Chen YW, Montaut S, Joly HA, Wang M, Belzile N. Synthesis, identification and chemical features of high-purity trimethylselenonium iodide. J Sulfur Chem. 2010; https://doi.org/10.1080/17415993.2010.516435
Eichler Š, Kaňa A, Kalousová M, Vosmanská M, Korotvička M, Zima T, Mestek O. Speciation analysis of selenium in human urine by liquid chromatography and inductively coupled plasma mass spectrometry for monitoring of selenium in body fluids. Chem Speciat Bioavailab. 2015; https://doi.org/10.1080/09542299.2015.1107502
Šlejkovec Z, Van Elteren JT, Woroniecka UD, Kroon KJ, Falnoga I, Byrne AR. Preliminary study on the determination of selenium compounds in some selenium-accumulating mushrooms. Biol Trace Elem Res. 2000. https://doi.org/10.1385/BTER:75:1-3:139.
Suwazono Y, Åkesson A, Alfvén T, Järup L, Vahter M. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005. https://doi.org/10.1080/13547500500159001.
Stajnko A, Šlejkovec Z, Mazej D, France-Stiglic A, Briški AS, Prpić I, Spirić Z, Horvat M, Falnoga I. Arsenic metabolites; selenium; and AS3MT, MTHFR, AQP4, AQP9, SELENOP, INMT, and MT2A polymorphisms in Croatian-Slovenian population from PHIME-CROME study. Environ Res. 2019. https://doi.org/10.1016/j.envres.2018.11.045.
Dedina J, Tsalev DL. Hydride generation atomic absorption spectrometry. New York: Wiley; 1995.
Bye R. Generation of selenium hydride from alkaline solutions: a new concept of the hydride generation-atomic absorption technique. J Automatic Chem. 1989;11:156–8.
D’Ulivo A, Marcucci K, Bramanti E, Lampugnani L, Zamboni R. Studies in hydride generation atomic fluorescence determination of selenium and tellurium. Part 1 - self interference effect in hydrogen telluride generation and the effect of KI. Spectrochim Acta Part B. 2000;55:1325–36.
Liu L, Zhang X, Yang L, Ren L, Wang D, Ye J. Metal nanoparticles induced photocatalysis. National Sci Rev. 2017. https://doi.org/10.1093/nsr/nwx019.
Suchorski Y, Rupprechter G. Heterogeneous surfaces as structure and particle size libraries of model catalysts. Catal Lett. 2018. https://doi.org/10.1007/s10562-018-2506-1.
Alaejos MS, Romero CD. Urinary selenium concentrations. Clin Chem. 1993;39:2040–52.
Lajin B, Francesconi K. The association between the urinary excretion of trimethylselenonium and trimethylsulfonium in humans. PLoS ONE. 2016. https://doi.org/10.1371/journal.pone.0167013.
Acknowledgements
The work was a part of a research program funded by the Slovenian Research Agency (P1-0143).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Sampling was performed following the ethical standards (Declaration of Helsinki) and written consent was obtained from all participants. The protocol was approved by the National Medical Ethics Committee of the Republic of Slovenia (number of accordance: 0120–431/2018/4).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Šlejkovec, Z., Stajnko, A., Mazej, D. et al. Trimethylselenonium ion determination in human urine by high-performance liquid chromatography–hydride generation–atomic fluorescence spectrometry optimization of the hydride generation step. Anal Bioanal Chem 415, 317–326 (2023). https://doi.org/10.1007/s00216-022-04408-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04408-6