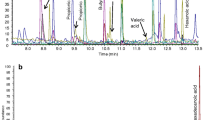
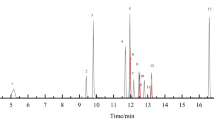
Abstract
The present research is focused on the optimization of an automatized sample preparation and fast gas chromatography–mass spectrometry (GC-MS) method for the analysis of fatty acid methyl esters (FAMEs) in blood samples and dietary supplements, with the primary objective being a significant reduction of the analysis time and, hence, an enhanced sample throughput. The mass spectrometer was operated in the scan/selected ion monitoring (SIM) acquisition method, thus enabling the obtainment of qualitative and (highly sensitive) quantitative data. The separation of FAMEs was obtained in about 11 min by using a micro-bore column of dimensions 15 m × 0.10 mm ID × 0.10 µm df with a polyethylene glycol stationary phase. The novelty of the research involves reducing analysis time by using the novel fast GC–MS method with increased identification reliability and sensitivity in a single chromatographic run. With regard to the figures of merit, linearity, accuracy, and limits of detection (LoD) and quantification (LoQ) were determined. Specifically, regression coefficients were between 0.9901 and 0.9996; the LoDs ranged from 0.05 to 1.02 µg g−1 for the blood analysis method, and from 0.05 to 0.26 mg g−1 in the case of the dietary supplement approach. With respect to LoQs, the values were in the ranges of 0.15–3.39 µg g−1 and 0.15–0.86 mg g−1 for blood and dietary supplements analysis methods, respectively. Accuracy was evaluated by analyzing certified reference materials (human plasma, fish oil).
Graphical abstract




Similar content being viewed by others
References
Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197(2):821–8.
Block RC, Harris WS, Reid KJ, Spertus JA. Omega-6 and trans fatty acids in blood cell membranes: a risk factor for acute coronary syndromes? Am Heart J. 2008;156(6):1117–23.
Harris WS. The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep. 2006;8(6):453–9.
Harris WS. Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol Res. 2007;55(3):217–23.
Külzow N, Witte AV, Kerti L, Grittner U, Schuchardt JP, Hahn A, et al. Impact of omega-3 fatty acid supplementation on memory functions in healthy older adults. J Alzheimers Dis. 2016;51(3):713–25.
Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66(2):237–59.
Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol. 2007;23(1).
Rigano F, Arena P, Mangraviti D, Donnarumma D, Dugo P, Donato P, et al. Identification of high-value generating molecules from the wastes of tuna fishery industry by liquid chromatography and gas chromatography hyphenated techniques with automated sample preparation. J Sep Sci. 2021;44(8):1571–80.
Shahidi F. Nutraceutical and specialty lipids and their co-products. 1st ed. Boca Raton, FL: CRC Press; 2006.
Shahidi F. Omega-3 fatty acids in health and disease. In: Hernandez EM, Hosokawa M, editors. Omega-3 oils - applications in functional foods. Urbana, Illinois: AOCS Press; 2011. p. 1–29.
Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fat Acids. 2007;77(5):327–35.
Yaqoob P, Pala HS, Cortina-Borja M, Newsholme EA, Calder PC. Encapsulated fish oil enriched in α-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur J Clin Invest. 2000;30:260–74.
Srigley CT, Rader JI. Content and composition of fatty acids in marine oil omega-3 supplements. J Agric Food Chem. 2014;62(29):7268–78.
Micalizzi G, Ragosta E, Farnetti S, Dugo P, Tranchida PQ, Mondello L, et al. Rapid and miniaturized qualitative and quantitative gas chromatography profiling of human blood total fatty acids. Anal Bioanal Chem. 2020;412(10):2327–37.
El-Hamdy AH, Christie W. Preparation of methyl esters of fatty acids with trimethylsulphonium hydroxid an appraisal. J Chromatogr A. 1993;630:438–41.
Galli C, Risé P, Ghezzi S, Marangoni F. Fast Determination of fatty acids in whole blood collected from fingertips: application to the assessment of fatty acid patterns (and various indexes) in population studies. World Rev Nutr Diet. 2009;100:35–45.
Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27(1):114–20.
Rizzo AM, Montorfano G, Negroni M, Adorni L, Berselli P, Corsetto P, et al. A rapid method for determining arachidonic:eicosapentaenoic acid ratios in whole blood lipids: correlation with erythrocyte membrane ratios and validation in a large Italian population of various ages and pathologies. Lipids Health Dis. 2010;9:7.
Certificate of Analysis, Standard Reference Material 1950 metabolites in human plasma (accessed October 06, 2021). Available from: https://www-s.nist.gov/srmors/view_cert.cfm?srm=1950.
Certificate of Analysis, Standard Reference Material 3275 Omega-3 and omega-6 fatty acids in fish oil (accessed October 06, 2021). Available from: https://www-s.nist.gov/srmors/quickSearch.cfm?srm=3275.
Blumberg LM, Klee MS. Optimal heating rate in gas chromatography. J Microcolumn Sep. 2000;12:508–14.
Leclercq PA, Novak J. Open tubular column. In: Quantitative analysis by gas chromatography, 2nd ed. Marcel Dekker Inc., New York; 1987. pp. 247–317.
Mondello L, Tranchida PQ, Dugo P, Dugo G. Rapid, micro-scale preparation and very fast gas chromatographic separation of cod liver oil fatty acid methyl esters. J Pharm Biomed Anal. 2006;5(41):1566–70.
Benner BA Jr, Schantz MM, Powers CD, Schleicher RL, Camara JE, Sharpless KE, et al. Standard reference material (SRM) 2378 fatty acids in frozen human serum. Certification of a clinical SRM based on endogenous supplementation of polyunsaturated fatty acids. Anal Bioanal Chem. 2018;410:2321–9.
Schantz MM, Powers CD, Schleicher RL, Betz JM, Wise SA. Interlaboratory analytical comparison of fatty acid concentrations in serum or plasma. Clin Chim Acta. 2016;462:148–52.
Ferreri C, Chatgilialoglu C. Membrane lipidomics for personalized health. 1st ed. Ltd: United Kingdom: John Wiley & Sons; 2015.
Guillou H, Zadravec D, Martin PGP, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010;49(2):186–99.
Sears B. The Omega RX Zone: the miracle of the new high-dose fish oil. United Kingdom: Harper Collins Publishers Ltd.; 2003.
Chee KM, Gong JX, Rees DM, Meydani M, Ausman L, Johnson J, et al. Fatty acid content of marine oil capsules. Lipids. 1990;25(9):523–8.
US Food and Drug Administration, Petition for a health claim for eicosapentaenoic acid and docosahexaenoic acid and reduction of blood pressure in the general population. 2019.
Ackman RG, Ratnayake WMN, Macpherson EJ. EPA and DHA contents of encapsulated fish oil products. J Am Oil Chem Soc. 1989;66(8):1162–4.
Acknowledgements
The authors thank Merck Life Science and Shimadzu Corporations for their continuous support. The research was conducted within the PRIN 2017 “Cutting-edge analytical chemistry methodologies and bio-tools to boost precision medicine in hormone-related diseases” supported by the Ministry University and Scientific Research, Prot. 2017Y2PAB8a. The scholarship of Ivan Aloisi was funded by the “Italian Ministry for the University and Research (MIUR)” within the National Operative Project “Dottorati Innovativi con caratterizzazione Industriale” (CCI 2014IT16M2OP005). This article is based upon work from the Sample Preparation Task Force and Network, supported by the Division of Analytical Chemistry of the European Chemical Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Statement of human rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1975 (in its most recently amended version).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The author Luigi Mondello is an editor of this journal but was not involved in the peer review of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferracane, A., Aloisi, I., Galletta, M. et al. Automated sample preparation and fast GC–MS determination of fatty acids in blood samples and dietary supplements. Anal Bioanal Chem 414, 8423–8435 (2022). https://doi.org/10.1007/s00216-022-04379-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04379-8