Abstract
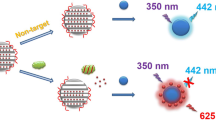
Exploring sensors based on Förster resonance energy transfer (FRET) systems enables the continuous development of biological sensing technologies. Herein, we report the construction of a FRET sensor with dual-emissive quantum dots (QDs) and meso-tetra(4-sulfonatophenyl) porphine (TSPP). The sensor is composed of mesial green-emissive QDs with a thick silica shell (gQD@SiO2) and circumjacent blue-emissive QDs coated with ultra-thin silica spacer, on which is linked TSPP (bQD@SiO2-TSPP). The gQD@SiO2 endows the sensor with a fluorescent background. Due to the ultra-thin silica spacing, coupled with the superior resonance effect of bQD fluorescence and the Soret-band absorption of TSPP, the FRET efficiency is highly sensitive to the chelation state of TSPP. Relying on the absorbance transition of TSPP complexed with Fe(III), the FRET sensor is applied for ultra-sensitive Fe(III) detection. In aqueous solution, the sensor is demonstrated to linearly detect Fe(III) in the range of 0–1 μM, with a limit of detection (LOD) of 40 nM. More importantly, reliable Fe(III) detection can be achieved via the specific complexation of Fe(III) by TSPP and the ratiometric fluorescent response. As such, the inter-/intra-day precisions in standard samples, as well as the recovery rate in biological matrices, are fully validated. The excellent analytical performance, in combination with the excellent biocompatibility of the FRET sensor, allows semi-quantitative Fe(III) imaging in living cells.
Graphical abstract








Similar content being viewed by others
References
Park JM, Hong KI, Lee H, Jang WD. Bioinspired applications of porphyrin derivatives. Acc Chem Res. 2021;54:2249–60.
Valicsek Z, Horvath O. Application of the electronic spectra of porphyrins for analytical purposes: the effects of metal ions and structural distortions. Microchem J. 2013;107:47–62.
Zhang XP, Lin B, Shu Y, Wang JH. “Switch-on” fluorescence sensing platform based on porphyrin metal-organic frameworks for rapid and specific detection of zinc ion. Anal Bioanal Chem. 2021;413:5161–8.
Ding YB, Zhu WH, Xie YS. Development of ion chemosensors based on porphyrin analogues. Chem Rev. 2017;117:2203–56.
Zhang XA, Lovejoy KS, Jasanoff A, Lippard SJ. Water-soluble porphyrins imaging platform for MM zinc sensing. P Natl Acad Sci USA. 2007;104:10780–5.
Almeda S, Gandolfi HE, Arce L, Valcarcel M. Potential of porphyrins as chromogenic reagents for determining metals in capillary electrophoresis. J Chromatogr A. 2009;1216:6256–8.
Monti D, Nardis S, Stefanelli M, Paolesse R, Di Natale C, D’Amico A. Porphyrin-based nanostructures for sensing applications. J Sensors. 2009;2009:856053.
Zhang WJ, Peng J, Lu LM, Zhang J, Zhang XB. 2-(2′-Hydroxyphenyl)-4(3H)-quinazolinone derivatives based fluorescent probes for mercury(II) via an intramolecular proton transfer mechanism. Int J Environ An Ch. 2012;92:810–20.
Qi DD, Zhang JH, Zhang DL, Zhu ML, Gong L, Su CR, Lu WX, Bian YZ, Jiang JZ. A phthalocyanine-porphyrin triad for ratiometric fluorescent detection of lead(II) ions. Dyes Pigments. 2020;173:107941.
Zhao LZ, Zhao YX, Li RS, Wu DH, Xu R, Li SS, Zhang YZ, Ye H, Xin QP. A porphyrin-based optical sensor membrane prepared by electrostatic self-assembled technique for online detection of cadmium(II). Chemosphere. 2020;238:124552.
Liu YQ, Seidi F, Deng C, Li RY, Xu TT, Xiao HN. Porphyrin derived dual-emissive carbon quantum dots: customizable synthesis and application for intracellular Cu2+ quantification. Sensor Actuat B-Chem. 2021;343:130072.
Qiao T, Parobek D, Son DH. Photons and charges from colloidal doped semiconductor quantum dots. J Mater Chem C. 2019;7:14788–97.
Chern M, Kays JC, Bhuckory S, Dennis AM. Sensing with photoluminescent semiconductor quantum dots. Methods Appl Fluores. 2019;7:012005.
Wang Y, Huo DQ, Wu HX, Liu H, Li JJ, Yang M, Huang CH, Hou CJ. A ratiometric fluorescent assay for fluazinam based on FRET between CdTe quantum dots and porphyrin. NANO. 2017;12:1750128.
Ingram JM, Zhang CF, Xu J, Schiff SJ. FRET excited ratiometric oxygen sensing in living tissue. J Neurosci Meth. 2013;214:45–51.
Liu YQ, Qu XJ, Guo QS, Sun QJ, Huang XB. QD-biopolymer-TSPP assembly as efficient BiFRET sensor for ratiometric and visual detection of zinc ion. ACS Appl Mater Interfaces. 2017;9:4725–32.
Rajora MA, Lou JWH, Zheng G. Advancing porphyrin’s biomedical utility via supramolecular chemistry. Chem Soc Rev. 2017;46:6433–69.
Managa M, Ngoy BP, Nyokong T. Photophysical properties and photodynamic therapy activity of a meso-tetra(4-carboxyphenyl)porphyrin tetramethyl ester-graphene quantum dot conjugate. New J Chem. 2019;43:4518–24.
Schlachter A, Asselin P, Harvey PD. Porphyrin-containing MOFs and COFs as heterogeneous photosensitizers for singlet oxygen-based antimicrobial nanodevices. ACS Appl Mater Interfaces. 2021;13:26651–72.
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM. The essential metals for humans: a brief overview. J Inorg Biochem. 2019;195:120–9.
Abbasi U, Abbina S, Gill A, Takuechi LE, Kizhakkedathu JN. Role of iron in the molecular pathogenesis of diseases and therapeutic opportunities. ACS Chem Biol. 2021;16:945–72.
Camarena V, Huff TC, Wang GF. Epigenomic regulation by labile iron. Free Radical Bio Med. 2021;170:44–9.
Farhadi S, Zabardasti A, Rahmati MH. Manganese(III) porphyrin covalently bound to sol-gel derived silica (Mn(III) porphyrinosilica): a reusable and green heterogeneous photocatalyst for oxidative decarboxylation of alpha-arylacetic acids with H2O2. J Chem Res. 2011(3):157–60.
Lee KH, Lee JH, Song WS, Ko H, Lee C, Lee JH, Yang H. Highly efficient, color-pure, color-stable blue quantum dot light-emitting devices. ACS Nano. 2013;7:7295–302.
Aubert T, Soenen SJ, Wassmuth D, Cirillo M, Van Deun R, Braeckmans K, Hens Z. Bright and stable CdSe/CdS@SiO2 nanoparticles suitable for long-term cell labeling. ACS Appl Mater Interfaces. 2014;6:11714–23.
Freeman R, Willner I. Optical molecular sensing with semiconductor quantum dots (QDs). Chem Soc Rev. 2012;41:4067–85.
Zhu HT, Wang LN, Jie XM, Liu DD, Cao YM. Improved interfacial affinity and CO2 separation performance of asymmetric mixed matrix membranes by incorporating postmodified MIL-53(Al). ACS Appl Mater Interfaces. 2016;8:22696–704.
Valicsek Z, Ottó HO. Application of the electronic spectra of porphyrins for analytical purposes: the effects of metal ions and structural distortions. Microchem J. 2013;107:47–62.
Rana M, Chowdhury P. L-Glutathione capped CdSeS/ZnS quantum dot sensor for the detection of environmentally hazardous metal ions. J Lumin. 2019;206:105–12.
Jimenez HR, Julve M, Faus J. A solution study of the protonation and deprotonation equilibria of 5,10,15,20-tetra(para-sulphonatophenyl)porphyrin. Stability constants of its magnesium(II), copper(II) and zinc(II) complexes. J Chem Soc, Dalton Trans. 1991;8:1945–9.
Li MF, Fang HB, Ji YF, Chen YC, He WJ, Guo ZJ. Rational design of ratiometric Fe3+ fluorescent probes based on FRET mechanism. Chem Res Chinese U. 2022;38:67–74.
Zhang QX, Sun Y, Liu ML, Liu Y. Selective detection of Fe3+ ions based on fluorescence MXene quantum dots via a mechanism integrating electron transfer and inner filter effect. Nanoscale. 2020;12:1826–32.
Fontecave M, Pierre JL. Iron: Metabolism, toxicity and therapy. Biochimie. 1993;75:767–73.
Wu L, Guo QS, Liu YQ, Sun QJ. Fluorescence resonance energy transfer-based ratiometric fluorescent probe for detection of Zn2+ using a dual-emission silica-coated quantum dots mixture. Anal Chem. 2015;87:5318–23.
Kielmann M, Senge MO. Molecular engineering of free-base porphyrins as ligands-the N-H center dot center dot center dot X binding motif in tetrapyrroles. Angew Chem Int Edit. 2019;58:418–41.
Ishii H, Koh H. Analytical application of porphyrins-I spectrophotometric determination of ultramicro amounts of copper with α, β, γ, δ-tetra-(3-N-methylpyridyl)porphine. Talanta. 1977;24:417–20.
Liu KK, Zhang LN, Zhu LN, Zhang R, Li XZ, Kong DM. A water-soluble, cationic bis-porphyrin exhibiting a more sensitive fluorescent response to Cu2+ relative to its monomeric counterpart. Sens Actuat B-Chem. 2017;247:179–87.
Ishi H, Tsuchiai H. Spectrophotometric and analogue derivative spectrophotometric determination of trace zinc with 5, 10, 15, 20-tetrakis(4-sulfophenyl)porphine in presence of high concentrations of cadmium. Anal Sci. 1987;3:229–33.
Huszank R, Horvath O. A heme-like, water-soluble iron(II) porphyrin: thermal and photoinduced properties, evidence for sitting-atop structure. Chem Commun. 2005(2):224–226.
Liu YQ, Ye MF, Ge QY, Qu XJ, Guo QS, Hu XY, Sun QJ. Ratiometric quantum dot-ligand system made by phase transfer for visual detection of double-stranded DNA and single-nucleotide polymorphism. Anal Chem. 2016;88:1768–74.
Acknowledgements
This research was funded by the Natural Science Foundation of China, grant number 81860317; Natural Science Foundation of Jiangsu Province, grant number BK20210630; and Science and Technology Department of Guizhou Province, grant number 20192821.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Hu, X., Liang, F. et al. A FRET sensor based on quantum dots–porphyrin assembly for Fe(III) detection with ultra-sensitivity and accuracy. Anal Bioanal Chem 414, 7741–7751 (2022). https://doi.org/10.1007/s00216-022-04305-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04305-y