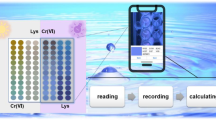
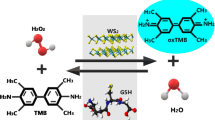
Abstract
Realizing the rapid and on-site detection of biothiols in complex biological and food samples using simple assays and devices remains a major challenge. In this study, biothiols containing sulfhydryl groups were found to be able to inhibit the photo-triggered oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB). Based on the discovery, using the commercially available and low-cost TMB as the chromogenic substrate, an enzyme-free colorimetric approach was developed for the rapid determination of biothiols. The method does not involve the introduction of any natural enzymes, nanoenzymes, and external oxidants. The mechanisms of the photoinduced oxidation of TMB and the detection of biothiols were proposed. Furthermore, a smartphone-based portable device integrated with test strips was constructed by the 3D printing technique. This device can simultaneously meet the requirements of the photocatalytic oxidation reaction of TMB and the detection of biothiols. The entire process only takes less than 5 min. The successful detection of cysteine in urine and milk samples demonstrates the great potential of the device in the on-site assays.
Graphical abstract






Similar content being viewed by others
References
Matuz-Mares D, Riveros-Rosas H, Vilchis-Landeros MM, Vázquez-Meza H. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants. 2021;10(8):1220. https://doi.org/10.3390/antiox10081220.
Rehman T, Shabbir MA, Inam-Ur-Raheem M, Manzoor MF, Ahmad N, Liu ZW, Ahmad MH, Siddeeg A, Abid M, Aadil RM. Cysteine and homocysteine as biomarker of various diseases. Food Sci Nutr. 2020;8(9):4696–707. https://doi.org/10.1002/fsn3.1818.
Ozben S, Kucuksayan E, Koseoglu M, Erel O, Neselioglu S, Ozben T. Plasma thiol/disulphide homeostasis changes in patients with relapsing-remitting multiple sclerosis. Int J Clin Pract. 2021;75(7):e14241. https://doi.org/10.1111/ijcp.14241.
Kaur N, Chopra S, Singh G, Raj P, Bhasin A, Sahoo SK, Kuwar A, Singh N. Chemosensors for biogenic amines and biothiols. J Mater Chem B. 2018;6(30):4872–902. https://doi.org/10.1039/C8TB00732B.
Luy J, Ameline D, Thobie-Gautier C, Boujtita M, Lebègue E. Detection of bacterial rhamnolipid toxin by redox liposome single impact electrochemistry. Angew Chem Int Ed. 2022;61(6):e202111416. https://doi.org/10.1002/anie.202111416.
Wang HB, Chen Y, Li Y, Liu YM. A sensitive fluorescence sensor for glutathione detection based on MnO2 nanosheets-copper nanoclusters composites. RSC Adv. 2016;6(83):79526–32. https://doi.org/10.1039/c6ra17850b.
Wang HB, Mao AL, Li YH, Gan T, Liu YM. A turn-on fluorescence strategy for biothiols determination by blocking Hg(II)-mediated fluorescence quenching of adenine-rich DNA-templated gold nanoclusters. Luminescence. 2020;35(8):1296–303. https://doi.org/10.1002/bio.3891.
Wang HB, Mao AL, Gan T, Liu YM. A turn-on fluorescence strategy for cellular glutathione determination based on the aggregation-induced emission enhancement of self-assembled copper nanoclusters. Analyst. 2020;145(21):7009–17. https://doi.org/10.1039/d0an01247e.
Lee TH, Lee CH, Alia NA, Liew RK, Hamdan N, Wong SL, Ong PY. Amino acid determination by HPLC combined with multivariate approach for geographical classification of Malaysian edible bird’s nest. J Food Compos Anal. 2022;107:104399. https://doi.org/10.1016/j.jfca.2022.104399.
Chang CW, Tseng WL. Gold nanoparticle extraction followed by capillary electrophoresis to determine the total, free, and protein-bound aminothiols in plasma. Anal Chem. 2010;82(7):2696–702. https://doi.org/10.1021/ac902342c.
Li F, Liu Y, Zhuang M, Zhang H, Liu X, Cui H. Biothiols as chelators for preparation of N-(aminobutyl)-N-(ethylisoluminol)/Cu2+ complexes bifunctionalized gold nanoparticles and sensitive sensing of pyrophosphate ion. ACS Appl Mater Interfaces. 2014;6(20):18104–11. https://doi.org/10.1021/am504985w.
Fernandes GM, Silva WR, Barreto DN, Lamarca RS, Gomes PCFL, Petruci JFD, Batista AD. Novel approaches for colorimetric measurements in analytical chemistry – a review. Anal Chim Acta. 2020;1135:187–203. https://doi.org/10.1016/j.aca.2020.07.030.
Kang B, Tang H, Gao M, Sun T, Zhao Z, Song S. Fluorescent hydrogel producing ZnO for colorimetric detection of glutathione and cysteine. Adv Mater Interfaces. 2021;8(20):2100765. https://doi.org/10.1002/admi.202100765.
Lu W, Chen J, Kong L, Zhu F, Feng Z, Zhan J. Oxygen vacancies modulation Mn3O4 nanozyme with enhanced oxidase-mimicking performance for L-cysteine detection. Sensor Actuator B Chem. 2021;333:129560. https://doi.org/10.1016/j.snb.2021.129560.
Chen Y, Chen T, Wu X, Yang G. CuMnO2 nanoflakes as pH-switchable catalysts with multiple enzyme-like activities for cysteine detection. Sensor Actuator B Chem. 2019;279:374–84. https://doi.org/10.1016/j.snb.2018.09.120.
Xian Z, Zhang L, Yu Y, Lin B, Wang Y, Guo M, Cao Y. Nanozyme based on CoFe2O4 modified with MoS2 for colorimetric determination of cysteine and glutathione. Microchim Acta. 2021;188(3):65. https://doi.org/10.1007/s00604-021-04702-7.
Duan W, Qiu Z, Cao S, Guo Q, Huang J, Xing J, Lu X, Zeng J. Pd–Fe3O4 Janus nanozyme with rational design for ultrasensitive colorimetric detection of biothiols. Biosens Bioelectron. 2022;196:113724. https://doi.org/10.1016/j.bios.2021.113724.
Su M, Chen H, Zhang H, Wang Z. Controllable bisubstrate multi-colorimetric assay based on peroxidase-like nanozyme and complementary colorharmonic principle for semi-quantitative detection of H2O2 with the naked eye. Microchim Acta. 2022;189(2):81. https://doi.org/10.1007/s00604-022-05169-w.
Bos ES, van der Doelen AA, van Rooy N, Schuurs AH. 3,3′,5,5′-Tetramethylbenzidine as an Ames test negative chromogen for horse-radish peroxidase in enzyme-immunoassay. J Immunoassay Immunochem. 1981;2(3–4):187–204. https://doi.org/10.1080/15321818108056977.
Ames BN, Kammen HO, Yamasaki E. Hair dyes are mutagenic: identification of a variety of mutagenic ingredients. Proc Natl Acad Sci U S A. 1975;72(6):2423–7. https://doi.org/10.1073/pnas.72.6.2423.
Voogd CE, Van der Stel JJ, Jacobs JJ. On the mutagenic action of some enzyme immunoassay substrates. J Immunol Methods. 1980;36(1):55–61. https://doi.org/10.1016/0022-1759(80)90093-9.
Zhang X, Li X, Lang Y, Wu P. Low-cost naked-eye UVB and UVC dosimetry based on 3,3′,5,5′-tetramethylbenzidine. Anal Chem. 2022;94(10):4373–9. https://doi.org/10.1021/acs.analchem.1c05190.
Bally RW, Gribnau TC. Some aspects of the chromogen 3,3′,5,5′-tetramethylbenzidine as hydrogen donor in a horseradish peroxidase assay. Clin Chem Lab Med. 1989;27(10):791–6. https://doi.org/10.1515/cclm.1989.27.10.791.
Foote CS. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54(5):659. https://doi.org/10.1111/j.1751-1097.1991.tb02071.x.
Wu S, Zhou R, Chen H, Zhang J, Wu P. Highly efficient oxygen photosensitization of carbon dots: the role of nitrogen doping. Nanoscale. 2020;12(9):5543–53. https://doi.org/10.1039/C9NR10986B.
Duan D, Fang X, Li K. A peroxidase-like nanoenzyme based on strontium(II)-ion-exchanged Prussian blue analogue derivative SrCoO3/Co3O4 nanospheres and carbon quantum dots for the colorimetric detection of tigecycline in river water. Talanta. 2022;240:123112. https://doi.org/10.1016/j.talanta.2021.123112.
Cao L, Yang C, Zhang B, Lv K, Li M, Deng K. Synergistic photocatalytic performance of cobalt tetra(2-hydroxymethyl-1,4-dithiin)porphyrazine loaded on zinc oxide nanoparticles. J Hazard Mater. 2018;359:388–95. https://doi.org/10.1016/j.jhazmat.2018.07.074.
Yuan C, Qin X, Xu Y, Li X, Chen Y, Shi R, Wang Y. Carbon quantum dots originated from chicken blood as peroxidase mimics for colorimetric detection of biothiols. J Photochem Photobiol A Chem. 2020;396:112529. https://doi.org/10.1016/j.jphotochem.2020.112529.
Du J, Wang J, Huang W, Deng Y, He Y. Visible light-activatable oxidase mimic of 9-mesityl-10-methylacridinium ion for colorimetric detection of biothiols and logic operations. Anal Chem. 2018;90(16):9959–65. https://doi.org/10.1021/acs.analchem.8b02197.
Lin M, Guo Y, Liang Z, Zhao X, Chen J, Wang Y. Simple and fast determination of biothiols using Fe3+-3,3′,5,5′-tetramethylbenzidine as a colorimetric probe. Microchem J. 2019;147:319–23. https://doi.org/10.1016/j.microc.2019.03.049.
Mackay AS, Payne RJ, Malins LR. Electrochemistry for the chemoselective modification of peptides and proteins. J Am Chem Soc. 2022;144(1):23–41. https://doi.org/10.1021/jacs.1c11185.
Simic MG, Jovanovic SV. Antioxidation mechanisms of uric acid. J Am Chem Soc. 1989;111(15):5778–82. https://doi.org/10.1021/ja00197a042.
Jocelyn PC. The standard redox potential of cysteine-cystine from the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur J Biochem. 1967;2(3):327–31. https://doi.org/10.1111/j.1432-1033.1967.tb00142.x.
Klotz LO, Kroncke KD, Sies H. Singlet oxygen-induced signaling effects in mammalian cells. Photochem Photobiol Sci. 2003;2(2):88–94. https://doi.org/10.1039/b210750c.
Seiwert B, Karst U. Simultaneous LC/MS/MS determination of thiols and disulfides in urine samples based on differential labeling with ferrocene-based maleimides. Anal Chem. 2007;79(18):7131–8. https://doi.org/10.1021/ac071016b.
Niero G, De Marchi M, Masi A, Penasa M, Cassandro M. Short communication: characterization of soluble thiols in bovine milk. J Dairy Sci. 2015;98(9):6014–7. https://doi.org/10.3168/jds.2015-9740.
Funding
This work was supported by the National Natural Science Foundation of China (21675056).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to participate
All individuals received a detailed description of the study and provided written informed consent to participate in the study before providing their urine samples. All of the experiments in this study were approved by the institutional ethics committee of South China Normal University.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 17749 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, J., Yu, Z., Fu, L. et al. Enzyme-free colorimetric detection of biothiols based on the photoinduced oxidation of 3,3′,5,5′-tetramethylbenzidine. Anal Bioanal Chem 414, 7731–7740 (2022). https://doi.org/10.1007/s00216-022-04304-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04304-z