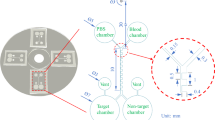
Abstract
Circulating tumor cells (CTCs), which have extremely low density in whole blood, are an important indicator of primary tumor metastasis. Isolation and enumeration of these cells are critical for clinical applications. Separation of CTCs from massive blood cells without labeling and addition of synthetic polymers is challenging. Herein, a novel well-defined co-flow microfluidic device is presented and used to separate CTCs in viscous blood by applying both inertial and viscoelastic forces. Diluted blood without any synthetic polymer and buffer solution were used as viscoelastic fluid and Newtonian fluid, respectively, and they were co-flowed in the designed chip to form a sheath flow. The co-flow system provides the function of particle pre-focusing and creates a tunable shear rate region at the interface to adjust the migration of particles or cells from the sample solution to the buffer solution. Successful separation of CTCs from viscous blood was demonstrated and enumeration was also conducted by image recognition after separation. The statistical results indicated that a recovery rate of cancer cells greater than 87% was obtained using the developed method, which proved that the direct separation of CTCs from diluted blood can be achieved without the addition of any synthetic polymer to prepare viscoelastic fluid. This method holds great promise for the separation of cells in viscous biological fluid without either complicated channel structures or the addition of synthetic polymers.
Graphical abstract








Similar content being viewed by others
References
Wan L, Liu Q, Liang D, Guo Y, Liu G, Ren J, He Y, Shan B. Circulating Tumor Cell and Metabolites as Novel Biomarkers for Early-Stage Lung Cancer Diagnosis. Front Oncol. 2021;11:630672. https://doi.org/10.3389/fonc.2021.630672.
Bidard FC, Proudhon C, Pierga JY. Circulating tumor cells in breast cancer. Mol Oncol. 2016;10(3):418–30. https://doi.org/10.1016/j.molonc.2016.01.001.
Gallo M, De Luca A, Maiello MR, D’Alessio A, Esposito C, Chicchinelli N, Forgione L, Piccirillo MC, Rocco G, Morabito A, Botti G, Normanno N. Clinical utility of circulating tumor cells in patients with non-small-cell lung cancer. Transl Lung Cancer Res. 2017;6(4):486–98. https://doi.org/10.21037/tlcr.2017.05.07.
Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E, Martel JM, Kojic N, Smith K, Chen PI, Yang J, Hwang H, Morgan B, Trautwein J, Barber TA, Stott SL, Maheswaran S, Kapur R, Haber DA, Toner M. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9(3):694–710. https://doi.org/10.1038/nprot.2014.044.
Shen S, Yi Z, Li X, Xie S, Shui L. Flow-Field-Assisted Dielectrophoretic Microchips for High-Efficiency Sheathless Particle/Cell Separation with Dual Mode. Anal Chem. 2021;93(21):7606–15. https://doi.org/10.1021/acs.analchem.1c00018.
Thomas RSW, Mitchell PD, Oreffo ROC, Morgan H, Green NG. Image-based sorting and negative dielectrophoresis for high purity cell and particle separation. Electrophoresis. 2019;40(20):2718–27. https://doi.org/10.1002/elps.201800489.
Li S, Li M, Bougot-Robin K, Cao W, Yeung Yeung Chau I, Li W, Wen W. High-throughput particle manipulation by hydrodynamic, electrokinetic, and dielectrophoretic effects in an integrated microfluidic chip. Biomicrofluidics. 2013;7(2):24106. https://doi.org/10.1063/1.4795856.
Li S, Li M, Hui YS, Cao W, Li W, Wen W. A novel method to construct 3D electrodes at the sidewall of microfluidic channel. Microfluid. Nanofluidics. 2012;14(3-4):499–508. https://doi.org/10.1007/s10404-012-1068-6.
Cao Q, Li Z, Wang Z, Qi F, Han X. Disaggregation and separation dynamics of magnetic particles in a microfluidic flow under an alternating gradient magnetic field. J. Phys. D. 2018;51(19):195002. https://doi.org/10.1088/1361-6463/aab9dd.
Nistler A, Niessner R, Seidel M. Magnetic nanocomposites: versatile tool for the combination of immunomagnetic separation with flow-based chemiluminescence immunochip for rapid biosensing of Staphylococcal enterotoxin B in milk. Anal Bioanal Chem. 2019;411(19):4951–61. https://doi.org/10.1007/s00216-019-01808-z.
Errarte A, Martin-Mayor A, Aginagalde M, Iloro I, Gonzalez E, Falcon-Perez JM, Elortza F, Bou-Ali MM. Thermophoresis as a technique for separation of nanoparticle species in microfluidic devices. Int J Therm Sci. 2020;156:106435. https://doi.org/10.1016/j.ijthermalsci.2020.106435.
Zhou Y, Yang C, Lam YC, Huang X. Thermophoresis of charged colloidal particles in aqueous media – Effect of particle size. Int. J. Heat Mass Transf. 2016;101:1283–91. https://doi.org/10.1016/j.ijheatmasstransfer.2016.05.109.
Ma Z, Collins DJ, Guo J, Ai Y. Mechanical Properties Based Particle Separation via Traveling Surface Acoustic Wave. Anal Chem. 2016;88(23):11844–51. https://doi.org/10.1021/acs.analchem.6b03580.
Zhang Y, Chen X. Particle separation in microfluidics using different modal ultrasonic standing waves. Ultrason Sonochem. 2021;75:105603. https://doi.org/10.1016/j.ultsonch.2021.105603.
Ahmed H, Destgeer G, Park J, Jung JH, Sung HJ. Vertical Hydrodynamic Focusing and Continuous Acoustofluidic Separation of Particles via Upward Migration. Adv Sci (Weinh). 2018;5(2):1700285. https://doi.org/10.1002/advs.201700285.
Sehgal P, Kirby BJ. Separation of 300 and 100 nm Particles in Fabry-Perot Acoustofluidic Resonators. Anal Chem. 2017;89(22):12192–200. https://doi.org/10.1021/acs.analchem.7b02858.
Jeon H, Jundi B, Choi K, Ryu H, Levy BD, Lim G, Han J, Fully-automated and field-deployable blood leukocyte separation platform using multi-dimensional double spiral (MDDS) inertial microfluidics. Lab Chip. 2020;20:3612-3624. https://doi.org/10.1039/d0lc00675k.
Condina MR, Dilmetz BA, Bazaz SR, Meneses J, Warkiani ME, Hoffmann P. Rapid separation and identification of beer spoilage bacteria by inertial microfluidics and MALDI-TOF mass spectrometry. Lab Chip. 2019; 19: 1961-1970. https://doi.org/10.1039/C9LC00152B.
Syed MS, Rafeie M, Vandamme D, Asadnia M, Henderson R, Taylor RA, Warkiani ME. Selective separation of microalgae cells using inertial microfluidics. Bioresour Technol. 2018;252:91–9. https://doi.org/10.1016/j.biortech.2017.12.065.
Zhang J, Yuan D, Zhao Q, Teo AJT, Yan S, Ooi CH, Li W, Nguyen NT. Fundamentals of Differential Particle Inertial Focusing in Symmetric Sinusoidal Microchannels. Anal Chem. 2019;91(6):4077–84. https://doi.org/10.1021/acs.analchem.8b05712.
Ren H, Zhu Z, Xiang N, Wang H, Zheng T, An H, Nguyen N-T, Zhang J. Multiplexed serpentine microchannels for high-throughput sorting of disseminated tumor cells from malignant pleural effusion. Sens. Actuators B Chem. 2021;337. https://doi.org/10.1016/j.snb.2021.129758.
Bilican I. Cascaded contraction-expansion channels for bacteria separation from RBCs using viscoelastic microfluidics. J Chromatogr A. 2021;1652:462366. https://doi.org/10.1016/j.chroma.2021.462366.
Mahboubidoust A, Ramiar A, Sedighi K. Design of an optimized ECCA microchannel for particle manipulation utilizing dean flow coupled elasto-inertial method. Adv Powder Technol. 2021;32(5):1688–709. https://doi.org/10.1016/j.apt.2021.03.029.
Fan L-L, Yan Q, Guo J, Zhao H, Zhao L, Zhe J. Inertial particle focusing in microchannels with gradually changing geometrical structures. J Micromech Microeng. 2017;27(1):015027. https://doi.org/10.1088/1361-6439/27/1/015027.
Sim TS, Kwon K, Park JC, Lee JG, Jung HI. Multistage-multiorifice flow fractionation (MS-MOFF): continuous size-based separation of microspheres using multiple series of contraction/expansion microchannels. Lab Chip. 2011;11(1):93–9. https://doi.org/10.1039/c0lc00109k.
Zhou J, Kulasinghe A, Bogseth A, O'Byrne K, Punyadeera C, Papautsky I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst Nanoeng. 2019;5:8. https://doi.org/10.1038/s41378-019-0045-6.
Lu X, Liu C, Hu G, Xuan X. Particle manipulations in non-Newtonian microfluidics: A review. J Colloid Interface Sci. 2017;500:182–201. https://doi.org/10.1016/j.jcis.2017.04.019.
Tian F, Zhang W, Cai L, Li S, Hu G, Cong Y, Liu C, Li T, Sun J. Microfluidic co-flow of Newtonian and viscoelastic fluids for high-resolution separation of microparticles. Lab Chip. 2017;17(18):3078–85. https://doi.org/10.1039/c7lc00671c.
Liu P, Liu H, Yuan D, Jang D, Yan S, Li M. Separation and Enrichment of Yeast Saccharomyces cerevisiae by Shape Using Viscoelastic Microfluidics. Anal Chem. 2021;93(3):1586–95. https://doi.org/10.1021/acs.analchem.0c03990.
Karimi A, Yazdi S, Ardekani AM. Hydrodynamic mechanisms of cell and particle trapping in microfluidics. Biomicrofluidics. 2013;7(2):21501. https://doi.org/10.1063/1.4799787.
Nam J, Namgung B, Lim CT, Bae JE, Leo HL, Cho KS, Kim S. Microfluidic device for sheathless particle focusing and separation using a viscoelastic fluid. J Chromatogr A. 2015;1406:244–50. https://doi.org/10.1016/j.chroma.2015.06.029.
Nam J, Yoon J, Jee H, Jang WS, Lim CS. High-Throughput Separation of Microvesicles from Whole Blood Components Using Viscoelastic Fluid. Adv. Mater. 2020;5(12):2000612. https://doi.org/10.1002/admt.202000612.
Tan J, Park SY, Leo HL, Kim S. Continuous Separation of White Blood Cells From Whole Blood Using Viscoelastic Effects. IEEE Trans Biomed Circuits Syst. 2017;99:1–7. https://doi.org/10.1109/TBCAS.2017.2748232.
Nam J, Jang WS, Lim CS. Non-electrical powered continuous cell concentration for enumeration of residual white blood cells in WBC-depleted blood using a viscoelastic fluid. Talanta. 2019;197:12–9. https://doi.org/10.1016/j.talanta.2018.12.102.
Shi X, Liu L, Cao W, Zhu G, Tan W. A Dean-flow-coupled interfacial viscoelastic fluid for microparticle separation applied in a cell smear method. Analyst. 2019;144(20):5934–46. https://doi.org/10.1039/c9an01070j.
Lim H, Back SM, Hwang MH, Lee DH, Choi H, Nam J. Sheathless High-Throughput Circulating Tumor Cell Separation Using Viscoelastic non-Newtonian Fluid. Micromachines (Basel). 2019;10(7):462. https://doi.org/10.3390/mi10070462.
Narayana IS, Kumar T, Martensson G, Russom A. High resolution and rapid separation of bacteria from blood using elasto-inertial microfluidics. Electrophoresis. 2021;42(23):2538–51. https://doi.org/10.1002/elps.202100140.
Liu C, Xue C, Chen X, Shan L, Tian Y, Hu G. Size-Based Separation of Particles and Cells Utilizing Viscoelastic Effects in Straight Microchannels. Anal Chem. 2015;87(12):6041–8. https://doi.org/10.1021/acs.analchem.5b00516.
Zhou J, Papautsky I. Viscoelastic microfluidics: progress and challenges. Microsyst Nanoeng. 2020;6:113. https://doi.org/10.1038/s41378-020-00218-x.
Thompson MS, Vadala TP, Vadala ML, Lin Y, Riffle JS. Synthesis and applications of heterobifunctional poly(ethylene oxide) oligomers. Polymer. 2008;49(2):345–73. https://doi.org/10.1016/j.polymer.2007.10.029.
Bodratti AM, Sarkar B, Alexandridis P. Adsorption of poly(ethylene oxide)-containing amphiphilic polymers on solid-liquid interfaces: Fundamentals and applications. Adv Colloid Interface Sci. 2017;244:132–63. https://doi.org/10.1016/j.cis.2016.09.003.
Liu X, Xu Y, Wu Z, Chen H. Poly(N-vinylpyrrolidone)-modified surfaces for biomedical applications. Macromol Biosci. 2013;13(2):147–54. https://doi.org/10.1002/mabi.201200269.
Berret JF. Local viscoelasticity of living cells measured by rotational magnetic spectroscopy. Nat Commun. 2016;7:10134. https://doi.org/10.1038/ncomms10134.
Brust M, Schaefer C, Doerr R, Pan L, Garcia M, Arratia PE, Wagner C. Rheology of Human Blood Plasma: Viscoelastic Versus Newtonian Behavior. Phys. Rev. Lett. 2013;110:078305. https://doi.org/10.1103/PhysRevLett.110.078305.
Indei T, Schieber JD, Córdoba A. Competing effects of particle and medium inertia on particle diffusion in viscoelastic materials, and their ramifications for passive microrheology. Phys. Rev. E. 2012;85(4). https://doi.org/10.1103/PhysRevE.85.041504.
Li D, Lu X, Xuan X. Viscoelastic Separation of Particles by Size in Straight Rectangular Microchannels: A Parametric Study for a Refined Understanding. Anal Chem. 2016;88(24):12303–9. https://doi.org/10.1021/acs.analchem.6b03501.
Manshadi MKD, Mohammadi M, Monfared LK, Sanati-Nezhad A. Manipulation of micro- and nanoparticles in viscoelastic fluid flows within microfluid systems. Biotechnol Bioeng. 2020;117(2):580–92. https://doi.org/10.1002/bit.27211.
Tian F, Cai L, Chang J, Li S, Liu C, Li T, Sun J. Label-free isolation of rare tumor cells from untreated whole blood by interfacial viscoelastic microfluidics. Lab Chip. 2018;18(22):3436–45. https://doi.org/10.1039/c8lc00700d.
Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y, Wei J, Hu G, Nie G, Sun J. Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS Nano. 2017;11(7):6968–76. https://doi.org/10.1021/acsnano.7b02277.
Jaensson NO, Mitrias C, Hulsen MA, Anderson PD. Shear-Induced Migration of Rigid Particles near an Interface between a Newtonian and a Viscoelastic Fluid. Langmuir. 2018;34(4):1795–806. https://doi.org/10.1021/acs.langmuir.7b03482.
Lu X, Xuan X. Inertia-Enhanced Pinched Flow Fractionation. Anal. Chem. 2015;87:4560–5. https://doi.org/10.1021/acs.analchem.5b00752.
Zhou J, Tu C, Liang Y, Huang B, Fang Y, Liang X, Papautsky I, Ye X. Isolation of cells from whole blood using shear-induced diffusion. Sci Rep. 2018;8(1):9411. https://doi.org/10.1038/s41598-018-27779-2.
Nam J, Lim H, Kim D, Jung H, Shin S. Continuous separation of microparticles in a microfluidic channel via the elasto-inertial effect of non-Newtonian fluid. Lab Chip. 2012;12(7):1347–54. https://doi.org/10.1039/c2lc21304d.
Acknowledgments
S. Li and Y. Xu would like to acknowledge the financial support from the National Key Research and Development Program of China (2020YFB2009001) and the National Natural Science Foundation of China (61904021, 62071072).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology, Shunbo Li and Yi Xu; Experiments, Mengnan Li and Chuang Ge; Data analysis, Mengnan Li and Yuping Yang; Validation, Minshan Gan and Li Chen; Writing, review & editing, all authors.
Corresponding authors
Ethics declarations
The studies were approved by the Chongqing University Cancer Hospital ethics committee (Ethical code: CZLS2021267-A) and were performed in accordance with ethical standards.
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1642 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Ge, C., Yang, Y. et al. Direct separation and enumeration of CTCs in viscous blood based on co-flow microchannel with tunable shear rate: a proof-of-principle study. Anal Bioanal Chem 414, 7683–7694 (2022). https://doi.org/10.1007/s00216-022-04299-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04299-7