Abstract
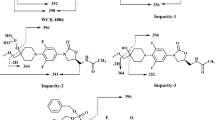
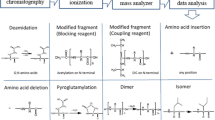
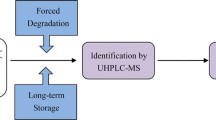
Cbf-14 (RLLRKFFRKLKKSV), a designed antimicrobial peptide derived from the cathelicidin family, is effective against drug-resistant bacteria. Structurally related peptide impurities in peptide medicines probably have side effects or even toxicity, thus impurity profiling research during the entire production process is indispensable. In this study, a simple liquid chromatography–high-resolution mass spectrometry (LC-HRMS) method using a quadrupole time-of-flight (Q-TOF) mass spectrometer was developed for separation, identification, and characterization of structurally related peptide impurities in Cbf-14. A total of one process-related impurity and thirty-two degradation products were identified, and seven of them have been synthesized and confirmed. These impurities have not been declared in custom synthetic peptides. The degradation products were divided into five categories: fifteen Cbf-14 hydrolysates, five Cbf-14 isomers, four acetyl-Cbf-14 isomers, two aldimine derivatives, and six oxidized impurities. Combined with the peptide synthesis and the stress-testing studies, the origins and the formation mechanisms of these impurities were elucidated, which provides a unique insight for the follow-up quality study of Cbf-14 and other peptide products.
Graphical abstract






Similar content being viewed by others
Abbreviations
- AMP:
-
Antimicrobial peptide
- SPPS:
-
Solid-phase peptide synthesis
- DIC:
-
N,N'-Diisopropylcarbodiimide
- AIMAOPA:
-
5-[(Aminoiminomethyl)amino]-2-oxopentanoic acid
- AIMG:
-
N-(Aminoiminomethyl)glutamine
- DACA:
-
2-Amino-4,5-dihydro-1H-1,3-diazepine-7-carboxylic acid
- PCA:
-
4,5-Dihydro-1H-pyrrole-2-carboxylic acid
- GSA:
-
Glutamic γ-semialdehyde
References
Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–62.
Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156:128–45.
Haney EF, Mansour SC, Hancock RE. Antimicrobial peptides: an introduction. Methods Mol Biol. 2017;1548:3–22.
Lazzaro BP, Zasloff M, Rolff J. Antimicrobial peptides: application informed by evolution. Science. 2020;368:487–95.
Wang YP, Hong J, Liu XH, Yang HL, Liu R, Wu J, et al. Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotics. PLoS ONE. 2008;3:e3217.
Zhou HM, Dou J, Wang J, Chen L, Wang H, Zhou W, et al. The antibacterial activity of BF-30 in vitro and in infected burned rats is through interference with cytoplasmic membrane integrity. Peptides. 2011;32:1131–8.
Tian YW, Wang H, Li B, Ke MY, Wang J, Dou J, et al. The cathelicidin-BF Lys16 mutant Cbf-K16 selectively inhibits non-small cell lung cancer proliferation in vitro. Oncol Rep. 2013;30:2502–10.
Ma L, Wang Y, Wang M, Tian Y, Kang W, Liu H, et al. Effective antimicrobial activity of Cbf-14, derived from a cathelin-like domain, against penicillin-resistant bacteria. Biomaterials. 2016;87:32–45.
Zhou CL, Jia YB, Tian YW, Li B, Li B, Wang H, et al. Polypeptide Cbf-14 resisting infection of drug-resistant bacteria and application. CN Patent 103435686B [P]. 2015–9–23.
Zhou CL, Han XR, Yu CZ, Ma LM, Dou J, Jia Y, B. Medical application of polypeptide Cbf-14 in resistance to fungal infection. CN Patent 105497872 [P]. 2016–4–20.
D’Hondt M, Bracke N, Taevernier L, Gevaert B, Verbeke F, Wynendaele E, et al. Related impurities in peptide medicines. J Pharm Biomed Anal. 2014;101:2–30.
Karongo R, Ikegami T, Stoll DR, Lämmerhofer M. A selective comprehensive reversed-phase×reversed-phase 2D-liquid chromatography approach with multiple complementary detectors as advanced generic method for the quality control of synthetic and therapeutic peptides. J Chromatogr A. 2020;1627:461430–41.
Choules MP, Bisson J, Simmler C, McAlpine JB, Giancaspro G, Bzhelyansky A, et al. NMR reveals an undeclared constituent in custom synthetic peptides. J Pharm Biomed Anal. 2020;178:112915.
Li M, Josephs RD, Daireaux A, Choteau T, Westwood S, Wielgosz RI, et al. Identification and accurate quantification of structurally related peptide impurities in synthetic human C-peptide by liquid chromatography-high resolution mass spectrometry. Anal Bioanal Chem. 2018;410:5059–70.
Cui LL, Yang ZC, Li M, Wei ZL, Fei Q, Huan YF, et al. Structural characterization of octreotide impurities by on-line electrochemistry-tandem mass spectrometry. Int J Mass Spectrom. 2019;435:18–25.
International Conference on Harmonisation (ICH) Guideline, Q1A stability testing of new drug substances and products, in: International Conference on Harmonisation, Geneva, Switzerland, 2003.
International Conference on Harmonisation (ICH) Guideline, Q5C stability testing of biotechnological/biological products, in: International Conference on Harmonisation, Geneva, Switzerland, 2011.
Tamizi E, Jouyban A. Forced degradation studies of biopharmaceuticals: selection of stress conditions. Eur J Pharm Biopharm. 2016;98:26–46.
National Committee of Pharmacopoeia. Pharmacopoeia of the People’s Republic of China. 2020th ed. Beijing: China Medical Science Press; 2020.
European Pharmacopoeia Committee, European Pharmacopoeia, 10th edition, European Directorate for the Quality of Medicines & HealthCare, France, 2020.
The United States Pharmacopieial Convention, USP 43-NF 38, The United States Pharmacopeial Convention, Rockville, MD, 2020.
Society of Japanese Pharmacopoeia, The Japanese Pharmacopoeia, 18th edition, Ministry of Health, Labour and Welfare, Tokyo, 2021.
Di Rocco M, Moloney M, Haren D, Gutierrez M, Earley S, Berendsen B, et al. Improving the chromatographic selectivity of β-lactam residue analysis in milk using phenyl-column chemistry prior to detection by tandem mass spectrometry. Anal Bioanal Chem. 2020;412:4461–75.
Marchand DH, Croes K, Dolan JW, Snyder LR, Henry RA, Kallury KM, et al. Column selectivity in reversed-phase liquid chromatography. VIII. Phenylalkyl and fluoro-substituted columns. J Chromatogr A. 2005;1062:65–78.
Han X, Wang DX. Recent progress in the improvement of the coupling efficiency of “difficult sequences” in peptide synthesis. ChemInform. 2008;39:111–7.
Mueller LK, Baumruck AC, Zhdanova H, Tietze AA. Challenges and perspectives in chemical synthesis of highly hydrophobic peptides. Front Bioeng Biotechnol. 2020;8:162–78.
D’Arrigo P, Arosio D, Cerioli L, Moscatelli D, Servi S, Viani F, et al. Base catalyzed racemization of amino acid derivatives. Tetrahedron Asymmetry. 2011;22:851–6.
Zhang JF, He QC, Lioy PJ. Characteristics of aldehydes: concentrations, sources, and exposures for indoor and outdoor residential microenviroments. Environ Sci Technol. 1994;28:146–52.
Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health. 2014;11:11192–200.
Li ML, Li HP, Huang HQ, Li YZ, Qin L, Xu X, et al. Identification and structural elucidation of a new cetrorelix methylene dimer impurity in cetrorelix acetate by using LC-MS/MS. J Pharm Biomed Anal. 2021;197:113946–51.
Acknowledgements
This work was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (Grant No. 2019ZX09201001-004-0052).
Author information
Authors and Affiliations
Contributions
Yitong Huo: methodology, investigation, formal analysis, writing the original draft; Kehui Xu: data curation, formal analysis, investigation; Yuting Lu: conceptualization; Lingman Ma: resources; Changlin Zhou: resources; Taijun Hang: supervision, writing—review and editing, project administration; Min Song: conceptualization, writing—review and editing, project administration.
Corresponding author
Ethics declarations
The experiments were conducted with synthetic Cbf-14 materials provided by the Department of Pharmaceutical Analysis of China Pharmaceutical University commercially sourced from GenScript (China).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huo, Y., Xu, K., Lu, Y. et al. Characterization of structurally related peptide impurities using HPLC-QTOF-MS/MS: application to Cbf-14, a novel antimicrobial peptide. Anal Bioanal Chem 414, 6485–6495 (2022). https://doi.org/10.1007/s00216-022-04205-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04205-1