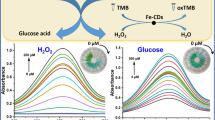
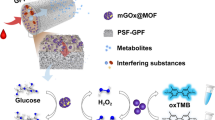
Abstract
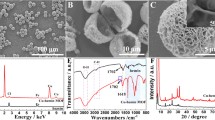
Covalent organic frameworks (COFs) with uniform porosity, good stability, and desired biocompatibility can function as carriers of immobilized enzymes. However, the obstructed pores or partially obstructed pores have hindered their applicability after loading enzymes. In this study, the hierarchical COFs were prepared as an ideal support to immobilize glucose oxidase (GOD) and obtain GOD@COF. The hierarchical porosity and porous structures of COFs provided sufficient sites to immobilize GOD and increased the rate of diffusion of substrate and product. Moreover, N,Fe-doped carbon dots (N,Fe-CDs) with peroxidase-like activity were introduced to combine with GOD@COF to construct an enzyme-mediated cascade reaction, which is the basis of the sensor GOD@COF/N,Fe-CDs. The sensor has been successfully built and applied to detect glucose. The limit of detection was 0.59 μM for determining glucose with the proposed fluorescence sensor. The practicability was illustrated by detecting glucose in human serum and saliva samples with satisfactory recoveries. The proposed sensor provided a novel strategy that introduced COF-immobilized enzymes for cascade reactions in biosensing and clinical diagnosis.
Graphical abstract








Similar content being viewed by others
References
Simon RC, Richter N, Busto E, Kroutil W. Recent developments of cascade reactions involving ω-transaminases. ACS Catal. 2013;4(1):129–43. https://doi.org/10.1021/cs400930v.
Tan W, Wei T, Huo J, Loubidi M, Liu T, Liang Y, Deng L. Electrostatic interaction-induced formation of enzyme-on-MOF as chemo-biocatalyst for cascade reaction with unexpectedly acid-stable catalytic performance. ACS Appl Mater Interfaces. 2019;11(40):36782–8. https://doi.org/10.1021/acsami.9b13080.
Chen W-H, Vázquez-González M, Zoabi A, Abu-Reziq R, Willner I. Biocatalytic cascades driven by enzymes encapsulated in metal–organic framework nanoparticles. Nat Catal. 2018;1(9):689–95. https://doi.org/10.1038/s41929-018-0117-2.
Pei X, Wu Y, Wang J, Chen Z, Liu W, Su W, Liu F. Biomimetic mineralization of nitrile hydratase into a mesoporous cobalt-based metal-organic framework for efficient biocatalysis. Nanoscale. 2020;12(2):967–72. https://doi.org/10.1039/c9nr06470b.
Oliveira FL, de Souza SP, Bassut J, Alvarez HM, Garcia-Basabe Y, Alves de Souza ROM, Esteves PM, Goncalves RSB. Enzyme-decorated covalent organic frameworks as nanoporous platforms for heterogeneous biocatalysis. Chemistry. 2019;25(69):15863–70. https://doi.org/10.1002/chem.201903807.
Magner E. Immobilisation of enzymes on mesoporous silicate materials. Chem Soc Rev. 2013;42(15):6213–22. https://doi.org/10.1039/c2cs35450k.
Li D, Fang Z, Duan H, Liang L. Polydopamine-mediated synthesis of core-shell gold@calcium phosphate nanoparticles for enzyme immobilization. Biomater Sci. 2019;7(7):2841–9. https://doi.org/10.1039/c9bm00283a.
Liang H, Jiang S, Yuan Q, Li G, Wang F, Zhang Z, Liu J. Co-immobilization of multiple enzymes by metal coordinated nucleotide hydrogel nanofibers: improved stability and an enzyme cascade for glucose detection. Nanoscale. 2016;8(11):6071–8. https://doi.org/10.1039/c5nr08734a.
Berijani K, Morsali A. The role of metal–organic porous frameworks in dual catalysis. Inorg Chem Front. 2021;8(15):3618–58. https://doi.org/10.1039/d1qi00394a.
Su D, Feng B, Xu P, Zeng Q, Shan B, Song Y. Covalent organic frameworks and electron mediator-based open circuit potential biosensor for in vivo electrochemical measurements. Anal Methods. 2018;10(35):4320–8. https://doi.org/10.1039/c8ay01386a.
Yan S, Guan X, Li H, Li D, Xue M, Yan Y, Valtchev V, Qiu S, Fang Q. Three-dimensional salphen-based covalent-organic frameworks as catalytic antioxidants. J Am Chem Soc. 2019;141(7):2920–4. https://doi.org/10.1021/jacs.9b00485.
Wang X, Han X, Zhang J, Wu X, Liu Y, Cui Y. Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J Am Chem Soc. 2016;138(38):12332–5. https://doi.org/10.1021/jacs.6b07714.
Wang Y, Zhuo S, Hou J, Li W, Ji Y. Construction of beta-cyclodextrin covalent organic framework-modified chiral stationary phase for chiral separation. ACS Appl Mater Interfaces. 2019;11(51):48363–9. https://doi.org/10.1021/acsami.9b16720.
Zhuo S, Wang X, Li L, Yang S, Ji Y. Chiral carboxyl-functionalized covalent organic framework for enantioselective adsorption of amino acids. ACS Appl Mater Interfaces. 2021;13(26):31059–65. https://doi.org/10.1021/acsami.1c09238.
Liu S, Li W-L, Zhang J-P. Revealing the potential application of chiral covalent organic frameworks in CO2 adsorption and separation. New J Chem. 2020;44(1):95–101. https://doi.org/10.1039/c9nj05172d.
Wang J, Yang X, Wei T, Bao J, Zhu Q, Dai Z. Fe-porphyrin-based covalent organic framework as a novel peroxidase mimic for a one-pot glucose colorimetric assay. ACS Appl Bio Mater. 2018;1(2):382–8. https://doi.org/10.1021/acsabm.8b00104.
Yue J-Y, Ding X-L, Wang L, Yang R, Bi J-S, Song Y-W, Yang P, Ma Y, Tang B. Novel enzyme-functionalized covalent organic frameworks for the colorimetric sensing of glucose in body fluids and drinks. Mater Chem Front. 2021;5(10):3859–66. https://doi.org/10.1039/d1qm00314c.
Sharma RK, Yadav P, Yadav M, Gupta R, Rana P, Srivastava A, Zbořil R, Varma RS, Antonietti M, Gawande MB. Recent development of covalent organic frameworks (COFs): synthesis and catalytic (organic-electro-photo) applications. Mater Horiz. 2020;7(2):411–54. https://doi.org/10.1039/c9mh00856j.
Gan J, Bagheri AR, Aramesh N, Gul I, Franco M, Almulaiky YQ, Bilal M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis—a review. Int J Biol Macromol. 2021;167:502–15. https://doi.org/10.1016/j.ijbiomac.2020.12.002.
Wang C, Liao K. Recent advances in emerging metal- and covalent-organic frameworks for enzyme encapsulation. ACS Appl Mater Interfaces. 2021;13(48):56752–76. https://doi.org/10.1021/acsami.1c13408.
Sun Q, Aguila B, Lan PC, Ma S. Tuning pore heterogeneity in covalent organic frameworks for enhanced enzyme accessibility and resistance against denaturants. Adv Mater. 2019;31(19):e1900008. https://doi.org/10.1002/adma.201900008.
Li Y, Zhou J, Wang L, Xie Z. Endogenous hydrogen sulfide-triggered MOF-based nanoenzyme for synergic cancer therapy. ACS Appl Mater Interfaces. 2020;12(27):30213–20. https://doi.org/10.1021/acsami.0c08659.
Li W, Li T, Chen S, Deng D, Ji Y, Li R (2022) Nanozyme-mediated cascade reaction system for ratiometric fluorescence detection of sarcosine. Sensors and Actuators B Chem 355. https://doi.org/10.1016/j.snb.2021.131341.
Wang CI, Chen WT, Chang HT. Enzyme mimics of Au/Ag nanoparticles for fluorescent detection of acetylcholine. Anal Chem. 2012;84(22):9706–12. https://doi.org/10.1021/ac300867s.
He S, Yang L, Balasubramanian P, Li S, Peng H, Kuang Y, Deng H, Chen W. Osmium nanozyme as peroxidase mimic with high performance and negligible interference of O2. J Mater Chem A. 2020;8(47):25226–34. https://doi.org/10.1039/d0ta09247a.
Dehvari K, Chiu SH, Lin JS, Girma WM, Ling YC, Chang JY. Heteroatom doped carbon dots with nanoenzyme like properties as theranostic platforms for free radical scavenging, imaging, and chemotherapy. Acta Biomater. 2020;114:343–57. https://doi.org/10.1016/j.actbio.2020.07.022.
Zhan Y, Zeng Y, Li L, Luo F, Qiu B, Lin Z, Guo L. Ratiometric fluorescent hydrogel test kit for on-spot visual detection of nitrite. ACS Sens. 2019;4(5):1252–60. https://doi.org/10.1021/acssensors.9b00125.
Zhan Y, Yang S, Luo F, Guo L, Zeng Y, Qiu B, Lin Z. Emission wavelength switchable carbon dots combined with biomimetic inorganic nanozymes for a two-photon fluorescence immunoassay. ACS Appl Mater Interfaces. 2020;12(27):30085–94. https://doi.org/10.1021/acsami.0c06240.
Shi W, Wang Q, Long Y, Cheng Z, Chen S, Zheng H, Huang Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun (Camb). 2011;47(23):6695–7. https://doi.org/10.1039/c1cc11943e.
Zhao D, Huang Y, Ouyang H, Shi B, Li S, Chen S, Zhao S. Facile preparation of Cu-doped carbon dots for naked-eye discrimination of phenylenediamine isomers and highly sensitive ratiometric fluorescent detection of H2O2. Talanta. 2021;239:123110. https://doi.org/10.1016/j.talanta.2021.123110.
Yue G, Li S, Liu W, Ding F, Zou P, Wang X, Zhao Q, Rao H. Ratiometric fluorescence based on silver clusters and N, Fe doped carbon dots for determination of H2O2 and UA: N, Fe doped carbon dots as mimetic peroxidase. Sens Actuators B Chem. 2019;287:408–15. https://doi.org/10.1016/j.snb.2019.02.060.
Gul U, Kanwal S, Tabassum S, Gilani MA, Rahim A. Microwave-assisted synthesis of carbon dots as reductant and stabilizer for silver nanoparticles with enhanced-peroxidase like activity for colorimetric determination of hydrogen peroxide and glucose. Mikrochim Acta. 2020;187(2):135. https://doi.org/10.1007/s00604-019-4098-x.
Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. https://doi.org/10.1007/s00125-018-4711-2.
Nordheim E, Geir Jenssen T. Chronic kidney disease in patients with diabetes mellitus. Endocr Connect. 2021;10(5):R151–9. https://doi.org/10.1530/EC-21-0097.
Arakawa T, Tomoto K, Nitta H, Toma K, Takeuchi S, Sekita T, Minakuchi S, Mitsubayashi K. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal Chem. 2020;92(18):12201–7. https://doi.org/10.1021/acs.analchem.0c01201.
Moyer J, Wilson D, Finkelshtein I, Wong B, Potts R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol Ther. 2012;14(5):398–402. https://doi.org/10.1089/dia.2011.0262.
Kurihara T, Lee D, Shinojima A, Kinoshita T, Nishizaki S, Arita Y, Hidaka Y, Nishi Y, Shirakawa Y, Kimura S, Tsuneyoshi Y, Torii H, Tsubota K, Negishi K. Glucose levels between the anterior chamber of the eye and blood are correlated based on blood glucose dynamics. PLoS ONE. 2021;16(9):e0256986. https://doi.org/10.1371/journal.pone.0256986.
Phiri MM, Mulder DW, Vorster BC (2019) Plasmonic detection of glucose in serum based on biocatalytic shape-altering of gold nanostars. Biosensors (Basel) 9(3). https://doi.org/10.3390/bios9030083.
Zhang H, Chen Z, Dai J, Zhang W, Jiang Y, Zhou A. A low-cost mobile platform for whole blood glucose monitoring using colorimetric method. Microchem J. 2021;162:105814. https://doi.org/10.1016/j.microc.2020.105814.
Lin M-H, Gupta S, Chang C, Lee C-Y, Tai N-H. Carbon nanotubes/polyethylenimine/glucose oxidase as a non-invasive electrochemical biosensor performs high sensitivity for detecting glucose in saliva. Microchem J. 2022;180:107547. https://doi.org/10.1016/j.microc.2022.107547.
Han H, He X, Wu M, Huang Y, Zhao L, Xu L, Ma P, Sun Y, Song D, Wang X. A novel colorimetric and near-infrared fluorescence probe for detecting and imaging exogenous and endogenous hydrogen peroxide in living cells. Talanta. 2020;217:121000. https://doi.org/10.1016/j.talanta.2020.121000.
Zhou R, Niu L, Hu Y, Qi Q, Huang W, Yang L. A novel dual-function fluorescent probe for the rapid detection of bisulfite and hydrogen peroxide in aqueous solution and living cells. Spectrochim Acta A Mol Biomol Spectrosc. 2021;248:119226. https://doi.org/10.1016/j.saa.2020.119226.
Zhou TY, Xu SQ, Wen Q, Pang ZF, Zhao X. One-step construction of two different kinds of pores in a 2D covalent organic framework. J Am Chem Soc. 2014;136(45):15885–8. https://doi.org/10.1021/ja5092936.
Yee YC, Hashim R, Mohd Yahya AR, Bustami Y (2019) Colorimetric analysis of glucose oxidase-magnetic cellulose nanocrystals (CNCs) for glucose detection. Sensors (Basel) 19(11). https://doi.org/10.3390/s19112511.
Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56(3):658–66. https://doi.org/10.1021/ja01318a036.
Sari N, Antepli E, Nartop D, Yetim NK. Polystyrene attached Pt(IV)-azomethine, synthesis and immmobilization of glucose oxidase enzyme. Int J Mol Sci. 2012;13(9):11870–80. https://doi.org/10.3390/ijms130911870.
Fereja TH, Kitte SA, Zafar MN, Halawa MI, Han S, Zhang W, Xu G. Highly sensitive and selective non-enzymatic glucose detection based on indigo carmine/hemin/H2O2 chemiluminescence. Analyst. 2020;145(3):1041–6. https://doi.org/10.1039/c9an02100k.
Jeong D, Lee W-Y (2021) Impedimetric detection of galactose based on a galactose-binding lectin, Ricinus communis agglutinin I (RCA120). J Electroanal Chem 903. https://doi.org/10.1016/j.jelechem.2021.115846.
Funding
This work has been supported by the Natural Science Foundation of Jiangsu Province (BE2019717).
Author information
Authors and Affiliations
Contributions
TL: methodology, conceptualization, writing—original draft. DD: formal analysis, verification. DDT: validation. SC: validation. YJ: supervision, funding acquisition, writing—review and editing. RL: supervision, funding acquisition, writing—review and editing.
Corresponding authors
Ethics declarations
Ethical standards and informed consent
The serum and saliva samples were collected at Nanjing Integrated Traditional Chinese and Western Medicine Hospital and China Pharmaceutical University and informed consents were obtained. The research was approved by the Research Ethics Committee of China Pharmaceutical University, and all experiments were performed in accordance with the guidelines and ethical standards of the institution.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, T., Deng, D., Tan, D. et al. Immobilized glucose oxidase on hierarchically porous COFs and integrated nanozymes: a cascade reaction strategy for ratiometric fluorescence sensors. Anal Bioanal Chem 414, 6247–6257 (2022). https://doi.org/10.1007/s00216-022-04197-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04197-y