Abstract
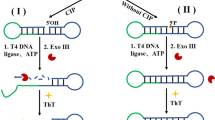
A universal enzyme strand (E-DNA) recyclable L-histidine (L-His), melamine (MA), and cisplatin (CP) biosensor was fabricated on the basis of a target-specific RNA-cleaving DNAzyme with specific auramine O (AuO) dye instead of thioflavin T. In this strategy, the substrate strand (S-DNA) of the RNA-cleaving site was constructed as an intramolecular stem-loop structure, and a GT-rich sequence was imprisoned in the double-stranded stem which inhibits the formation of stable G-quadruplex (G4cpx) with AuO. The presence of L-His initiates a catalytic reaction for cleaving the RNA site of the S-DNA hydrolytically releasing the GT-rich portion, which subsequently combines with AuO and forms a G4cpx for enhanced fluorescent signal. The subsequent addition of MA uncoils the G4cpx to form T-MA-T dsDNA, or addition of CP unwound the G4cpx to form CP-DNA leading to an intensive decrease of AuO emission. Remarkably, the liberated L-His can ultimately cause several rounds of cleavage, and the liberated E-DNA can catalyze the subsequent reaction with the other S-DNA. The use of L-His and E-DNA repeatedly induces S-DNA cleavage and intensifies the emission signal. The results show that the proposed biosensor is extremely sensitive to L-His, MA, and CP with a detection limit of 0.98, 10, and 3.4 nM respectively. To the best of our knowledge, the utilization of AuO as the G4cpx inducer and stabilizer for L-His, MA, and CP detection in real milk and urine samples has never been reported.










Similar content being viewed by others
References
Wu H, Tian D, Fan X, Fan W, Zhang Y, Jiang S, Wen C, Ma Q, Chen N, Xie X. Highly efficient production of L-histidine from glucose by metabolically engineered Escherichia coli. ACS Synth Biol. 2020;9:1813–22.
Zheng X, Yao T, Zhu Y, Shi S. Cu2+ modulated silver nanoclusters as an on-off-on fluorescence probe for the selective detection of l-histidine. Biosens Bioelectron. 2015;66:103–8.
Zhou N, Zhou Q, Meng G, Huang Z, Ke Y, Liu J, Wu N. Incorporation of a basil-seed-based surface enhanced Raman scattering sensor with a pipet for detection of melamine. ACS Sensors. 2016;1:1193–7.
Jeong S, Kwon WY, Hwang SH, Shin J, Kim Y, Lee M, Park KS. Fluorescence, turn-on detection of melamine based on its dual functions as fluorescence enhancer of DNA-AgNCs and Hg(II)-scavenger. Artif Cells Nanomed Biotechnol. 2019;47:621–5.
Hazra C, Adusumalli VNKB, Mahalingam V. 3,5-Dinitrobenzoic acid-capped upconverting nanocrystals for the selective detection of melamine. ACS Appl Mater Interfaces. 2014;6:7833–9.
Mandriota G, Di Corato R, Benedetti M, De Castro F, Fanizzi FP, Rinaldi R. Design and application of cisplatin-loaded magnetic nanoparticle clusters for smart chemotherapy. ACS Appl Mater Interfaces. 2019;11:1864–75.
Ma F, Yan S, Zhang J, Wang Y, Wang L, Wang Y, Zhang S, Du X, Zhang P, Chen HY, Huang S. Nanopore sequencing accurately identifies the cisplatin adduct on DNA. ACS Sens. 2021;6:3082–92.
Tateda N, Matsuhisa K, Hasebe K, Kitajima N, Miura T. High-performance liquid chromatographic method for rapid and highly sensitive determination of histidine using postcolumn fluorescence detection with o-phthaldialdehyde. J Chromatogr B Biomed Appl. 1998;718:235–41.
Venkatasami G, Sowa JR. A Rapid, Acetonitrile-free, HPLC method for determination of melamine in infant formula. Anal Chim Acta. 2010;665:227–30.
Lopez-Flores A, Jurado R, Garcia-Lopez P. A high-performance liquid chromatographic assay for determination of cisplatin in plasma, cancer cell, and tumor samples. J Pharmacol Toxicol Methods. 2005;52:366–72.
Bhawawet N, Fuangswasdi S, Imyim A. Determination of melamine based on the formation of an insoluble copper-melamine complex and flame atomic absorption spectrometry. Anal Lett. 2014;47:1931–7.
Raghavan R, Mulligan JA. Low-level (PPB) determination of cisplatin in cleaning validation (rinse water) samples. I. An Atomic Absorption Spectrophotometric Method. Drug Dev Ind Pharm. 2000;26:423–8.
Danielsen M, Nebel C, Dalsgaard TK. Simultaneous determination of L- and D-amino acids in proteins: a sensitive method using hydrolysis in deuterated acid and liquid chromatography–tandem mass spectrometry analysis. Foods. 2020;9:309.
Yang SP, Hu B, Li JQ, Han J, Zhang X, Chen HW, Liu Q, Liu QJ, Zheng J. Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct detection melamine in powdered milk products. Fenxi Huaxue/Chin J Anal Chem. 2009;37:691–4.
Ming X, Groehler A, Michaelson-Richie ED, Villalta PW, Campbell C, Tretyakova NY. Mass spectrometry based proteomics study of cisplatin-induced DNA-protein cross-linking in human fibrosarcoma (HT1080) cells. Chem Res Toxicol. 2017;30:980–95.
Gholivand MB, Ahmadi E, Mavaei M. A novel voltammetric sensor based on graphene quantum dots-thionine/nano-porous glassy carbon electrode for detection of cisplatin as an anti-cancer drug. Sensors Actuators B Chem. 2019;299:126975.
Zhang Z, Hu Y, Zhang H, Luo L, Yao S. Layer-by-layer assembly sensitive electrochemical sensor for selectively probing l-histidine based on molecular imprinting sol-gel at functionalized indium tin oxide electrode. Biosens Bioelectron. 2010;26:696–702.
Liang X, Wei H, Cui Z, Deng J, Zhang Z, You X, Zhang XE. Colorimetric detection of melamine in complex matrices based on cysteamine-modified gold nanoparticles. Analyst. 2011;136:179–83.
Chuaychob S, Thammakhet-Buranachai C, Kanatharana P, Thavarungkul P, Buranachai C, Fujita M, Maeda M. A nanobiosensor for the simple detection of small molecules using non-crosslinking aggregation of gold nanoparticles with G-quadruplexes. Anal Methods. 2020;12:230–8.
Pavadai R, Amalraj A, Subramanian S, Perumal P. High catalytic activity of fluorophore-labeled Y-shaped DNAzyme/3D MOF-MoS2NBs as a versatile biosensing platform for the simultaneous detection of Hg2+, Ni2+, and Ag+ ions. ACS Appl Mater Interfaces. 2021;13:31710–24.
Arunjegan A, Rajaji P, Sivanesan S, Panneerselvam P. A Turn-ON fluorometric biosensor based on SsDNA immobilized with a metal phenolic nanomaterial for the sequential detection of Pb(II) and epirubicin cancer drug. RSC Adv. 2021;11:12361–73.
Amalraj A, Pavadai R, Perumal P. Recyclable target metal-enhanced fluorometric naked eye aptasensor for the detection of Pb2+ and Ag+ ions based on the structural change of CaSnO3 @PDANS-constrained GC-rich SsDNA. ACS Omega. 2021;6:30580–97.
Rajaji P, Panneerselvam P. A novel polydopamine grafted 3D MOF nanocubes mediated GR-5/GC DNAzyme complex with enhanced fluorescence emission response toward spontaneous detection of Pb(II) and Ag(I) ions. ACS Omega. 2020;5:25188–98.
Amdursky N, Huppert D. Auramine-O as a fluorescence marker for the detection of amyloid fibrils. J Phys Chem B. 2012;116:13389–95.
Aydinoglu S, Pasti A, Biver T, Mennucci B. Auramine O interaction with DNA: a combined spectroscopic and TD-DFT analysis. Phys Chem Chem Phys. 2019;21:20606–12.
Ravikumar A, Panneerselvam P, Radhakrishnan K. Fluorometric determination of lead (II) and mercury (II) based on their interaction with a complex formed between graphene oxide and a DNAzyme. Microchim Acta. 2017;2:8–15.
Xu H, Geng F, Wang Y, Xu M, Lai X, Qu P, Zhang Y, Liu B. A label-free fluorescent molecular switch for a DNA hybridization assay utilizing a G-quadruplex-selective auramine O. Chem Commun. 2015;51:8622–5.
Xie Z, Lei J, Yang M, Li Y, Geng X, Liu S, Wang J. Conical nanofluidic channel for selective quantitation of melamine in combination with β-cyclodextrin and a single-walled carbon nanotube. Biosens Bioelectron. 2019;127:200–6.
N’Soukpo-Kossi CN, Descôteaux C, Asselin É, Tajmir-Riahi HA, Bérubé G. DNA interaction with novel antitumor estradiol-platinum(II) hybrid molecule: a comparative study with cisplatin drug. DNA Cell Biol. 2008;27:101–7.
Zhang C, Zhang HL, Han MN, Yang XL, Pei CH, Xu ZD, Du J, Li W, Chen S. DNA-affibody nanoparticle delivery system for cisplatin-based breast cancer chemotherapy. RSC Adv. 2019;9:1982–9.
Acknowledgements
The authors acknowledge the “Selective Excellence Research Initiative Fund SRMIST-2021” from the Department of Chemistry, SRM Institute of Science and Technology, Tamil Nadu—603 203, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amalraj, A., Perumal, P. Label-free DNAzyme for highly sensitive detection of multiple biomolecules in real samples through target-triggered catalytic cleavage reactions with auramine O’s discriminated fluorescence emission. Anal Bioanal Chem 414, 4021–4037 (2022). https://doi.org/10.1007/s00216-022-04061-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04061-z