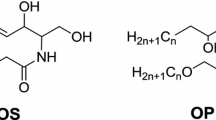
Abstract
The presence of a new ceramide subclass, the 1-O-acyl omega-linoleoyloxy ceramides [1-O-E (EO) Cer], has been previously highlighted in reconstructed human epidermis (RHE). These ceramides are double esterified on two positions. The first is the 1-O position of the sphingoid base moiety with a long to very long chain of acyl residues (1-O-E), and the second is the position of the ω-hydroxyl group of the fatty acid moiety with linoleic acid (EO). Considering its chemical structure and hydrophobicity, this subclass can contribute to the skin barrier. Thus, it is important to determine whether this subclass is also present in native human stratum corneum (SC). This work compares ceramide structures of this novel subclass between RHE (in vitro) and two sources of human SC (in vivo and ex vivo) using normal-phase high-performance liquid chromatography coupled to high-resolution mass spectrometry (NP-HPLC/HR-MSn). The results confirm the presence of this double esterified ceramide subclass [1-O-E (EO) Cer] in human SC. The molecular profile obtained from the RHE was very close to that found in the human SC (in vivo and ex vivo). In addition, thanks to the targeted MS2/MS3 analysis, a new ceramide subclass was discovered and characterized in the three studied samples. We propose to name it [A-1-O-E (EO) Cer] because in these ceramides species, the fatty acid—esterified with the sphingoid base on the 1-O position—is hydroxylated on the α position. These results highlight the potential of both the analytical method and the characterization approach employed in this study.
Graphical abstract





Similar content being viewed by others
Abbreviations
- APPI:
-
Atmospheric pressure photoionization
- Cer:
-
Ceramide
- FFAs:
-
Free fatty acids
- HR-MSn :
-
High-resolution mass spectrometry
- LC:
-
Liquid chromatography
- NP-HPLC:
-
Normal-phase high-performance liquid chromatography
- RHE:
-
Reconstructed human epidermis
- RT:
-
Retention time
- SC:
-
Stratum corneum
References
Madison KC. Barrier function of the skin: "la raison d’etre" of the epidermis. J Invest Dermatol. 2003;121(2):231–41. https://doi.org/10.1046/j.1523-1747.2003.12359.x.
Jiang SJ, Chen JY, Lu ZF, Yao J, Che DF, Zhou XJ. Biophysical and morphological changes in the stratum corneum lipids induced by UVB irradiation. J Dermatol Sci. 2006;44(1):29–36. https://doi.org/10.1016/j.jdermsci.2006.05.012.
Bouwstra JA, Dubbelaar FE, Gooris GS, Ponec M. The lipid organisation in the skin barrier. Acta Derm-Venereol Supplementum. 2000;208:23–30. https://doi.org/10.1080/000155500750042826.
Ansari MN, Nicolaides N, Fu HC. Fatty acid composition of the living layer and stratum corneum lipids of human sole skin epidermis. Lipids. 1970;5(10):838–45. https://doi.org/10.1007/BF02531977.
Norlen L, Nicander I, Lundsjo A, Cronholm T, Forslind B. A new HPLC-based method for the quantitative analysis of inner stratum corneum lipids with special reference to the free fatty acid fraction. Arch Dermatol Res. 1998;290(9):508–16. https://doi.org/10.1007/s004030050344.
van Smeden J, Bouwstra JA. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Current Prob Dermatol. 2016;49:8–26. https://doi.org/10.1159/000441540.
van Smeden J, Boiten WA, Hankemeier T, Rissmann R, Bouwstra JA. Vreeken RJ (2014) Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim Biophys Acta. 1841;1:70–9. https://doi.org/10.1016/j.bbalip.2013.10.002.
Rabionet M, Gorgas K. Sandhoff R (2014) Ceramide synthesis in the epidermis. Bba-Mol Cell Biol L. 1841;3:422–34. https://doi.org/10.1016/j.bbalip.2013.08.011.
Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009;91(6):784–90. https://doi.org/10.1016/j.biochi.2009.04.001.
Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, Homma R, Ishikawa J, Takagi Y, Kitahara T, Takema Y, Kita K. Characterization of overall ceramide species in human stratum corneum. J Lipid Res. 2008;49(7):1466–76. https://doi.org/10.1194/jlr.M800014-JLR200.
Rabionet M, Bayerle A, Marsching C, Jennemann R, Grone HJ, Yildiz Y, Wachten D, Shaw W, Shayman JA, Sandhoff R. 1-O-acylceramides are natural components of human and mouse epidermis. J Lipid Res. 2013;54(12):3312–21. https://doi.org/10.1194/jlr.M040097.
Assi A, Bakar J, Libong D, Sarkees E, Solgadi A, Baillet-Guffroy A, Michael-Jubeli R, Tfayli A. Comprehensive characterization and simultaneous analysis of overall lipids in reconstructed human epidermis using NPLC/HR-MS(n): 1-O-E (EO) Cer, a new ceramide subclass. Analytic bioanalytic Chem. 2020;412(3):777–93. https://doi.org/10.1007/s00216-019-02301-3.
Rampler E, Abiead YE, Schoeny H, Rusz M, Hildebrand F, Fitz V, Koellensperger G. Recurrent Topics in Mass Spectrometry-Based Metabolomics and Lipidomics-Standardization, Coverage, and Throughput. Analytic Chem. 2021;93(1):519–45. https://doi.org/10.1021/acs.analchem.0c04698.
Knox S, O’Boyle NM. Skin lipids in health and disease: A review. Chem Phys Lipids. 2021;236:105055. https://doi.org/10.1016/j.chemphyslip.2021.105055.
Berdyshev E, Goleva E, Bronova I, Dyjack N, Rios C, Jung J, Taylor P, Jeong M, Hall CF, Richers BN, Norquest KA, Zheng T, Seibold MA, Leung DY. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight. 2018;3(4). https://doi.org/10.1172/jci.insight.98006
Behne M, Uchida Y, Seki T, de Montellano PO, Elias PM, Holleran WM. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J Invest Dermatol. 2000;114(1):185–92. https://doi.org/10.1046/j.1523-1747.2000.00846.x.
Lee JY, Liu KH, Cho Y, Kim KP. Enhanced Triacylglycerol Content and Gene Expression for Triacylglycerol Metabolism, Acyl-Ceramide Synthesis, and Corneocyte Lipid Formation in the Epidermis of Borage Oil Fed Guinea Pigs. Nutrients. 2019;11(11). https://doi.org/10.3390/nu11112818
van Smeden J, Hoppel L, van der Heijden R, Hankemeier T, Vreeken RJ, Bouwstra JA. LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J Lipid Res. 2011;52(6):1211–21. https://doi.org/10.1194/jlr.M014456.
Danso M, Boiten W, van Drongelen V, Gmelig Meijling K, Gooris G, El Ghalbzouri A, Absalah S, Vreeken R, Kezic S, van Smeden J, Lavrijsen S, Bouwstra J. Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J Dermatol Sci. 2017;88(1):57–66. https://doi.org/10.1016/j.jdermsci.2017.05.005.
Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, Kitahara T, Takema Y, Koyano S, Yamazaki S, Hatamochi A. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130(10):2511–4. https://doi.org/10.1038/jid.2010.161.
Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993;1182(2):147–51. https://doi.org/10.1016/0925-4439(93)90135-n.
Kligman AM, Christophers E. Preparation of Isolated Sheets of Human Stratum Corneum. Arch Dermatol. 1963;88:702–5. https://doi.org/10.1001/archderm.1963.01590240026005.
Quatela A, Tfayli A, Baillet-Guffroy A. Examination of the effect of Stratum Corneum isolation process on the integrity of the barrier function: a confocal Raman spectroscopy study. Skin Res Technol: Off J Int Soc Bioeng Skin. 2016;22(1):75–80. https://doi.org/10.1111/srt.12231.
Folch J, Ascoli I, Lees M, Meath JA, Le BN. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951;191(2):833–41.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–7. https://doi.org/10.1139/o59-099.
Pedrosa TDN, Catarino CM, Pennacchi PC, Assis SR, Gimenes F, Consolaro MEL, Barros SBM, Maria-Engler SS. A new reconstructed human epidermis for in vitro skin irritation testing. Toxicol in vitro: Int J Pub Assoc BIBRA. 2017;42:31–7. https://doi.org/10.1016/j.tiv.2017.03.010.
Brohem CA, Cardeal LB, Tiago M, Soengas MS, Barros SB, Maria-Engler SS. Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res. 2011;24(1):35–50. https://doi.org/10.1111/j.1755-148X.2010.00786.x.
Acknowledgements
Financial support: The authors would like to thank the SILAB - Jean PAUFIQUE Corporate Foundation for financial support for this work. We would like to thank the Platform for the Analysis of Drugs and Metabolites (SAMM) at the University of Paris-Saclay for the technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Human skin was acquired with donor consent and with certified ethical procedures from patients undergoing cosmetic surgery at Rangueil Hospital, Toulouse, France.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bakar, J., Michael-Jubeli, R., Libong, D. et al. Stratum corneum ceramide profiles in vitro, ex vivo, and in vivo: characterization of the α-hydroxy double esterified ceramides. Anal Bioanal Chem 414, 3675–3685 (2022). https://doi.org/10.1007/s00216-022-04011-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04011-9