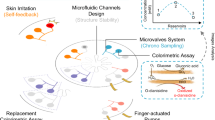
Abstract
A soft and flexible wearable sweat epidermal microfluidic device capable of simultaneously stimulating, collecting, and electrochemically analyzing sweat is demonstrated. The device represents the first system integrating an iontophoretic pilocarpine delivery system around the inlet channels of epidermal polydimethylsiloxane (PDMS) microfluidic device for sweat collection and analysis. The freshly generated sweat is naturally pumped into the fluidic inlet without the need of exercising. Soft skin-mounted systems, incorporating non-invasive, on-demand sweat sampling/analysis interfaces for tracking target biomarkers, are in urgent need. Existing skin conformal microfluidic-based sensors for continuous monitoring of target sweat biomarkers rely on assays during intense physical exercising. This work demonstrates the first example of combining sweat stimulation, through transdermal pilocarpine delivery, with sample collection through a microfluidic channel for real-time electrochemical monitoring of sweat glucose, in a fully integrated soft and flexible multiplexed device which eliminates the need of exercising. The on-body operational performance and layout of the device were optimized considering the fluid dynamics and evaluated for detecting sweat glucose in several volunteers. Furthermore, the microfluidic monitoring device was integrated with a real-time wireless data transmission system using a flexible electronic board PCB conformal with the body. The new microfluidic platform paves the way to real-time non-invasive monitoring of biomarkers in stimulated sweat samples for diverse healthcare and wellness applications.
Graphical abstract





Similar content being viewed by others
References
Yang Y, Gao W. Wearable and flexible electronics for continuous molecular monitoring. Chem Soc Rev. 2019;48:1465–91. https://doi.org/10.1039/c7cs00730b.
Kim J, Jeerapan I, Sempionatto JR, Barfidokht A, Mishra RK, Campbell AS, et al. Wearable bioelectronics: enzyme-based body-worn electronic devices. Acc Chem Res. 2018;51:2820–8. https://doi.org/10.1021/acs.accounts.8b00451.
Matzeu G, Florea L, Diamond D. Advances in wearable chemical sensor design for monitoring biological fluids. Sensors Actuators, B Chem. 2015;211:403–18. https://doi.org/10.1016/j.snb.2015.01.077.
Yang Y, Song Y, Bo X, Min J, Pak OS, Zhu L, et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat Biotechnol. 2020;38:217–24. https://doi.org/10.1038/s41587-019-0321-x.
Sempionatto JR, Khorshed AA, Ahmed A, De Loyola E Silva AN, Barfidokht A, Yin L, et al. Epidermal enzymatic biosensors for sweat vitamin C: toward personalized nutrition. ACS Sensors. 2020;5:1804–13. https://doi.org/10.1021/acssensors.0c00604.
Cheng Y, Wang K, Xu H, Li T, Jin Q, Cui D. Recent developments in sensors for wearable device applications. Anal Bioanal Chem. 2021;413:6037–57. https://doi.org/10.1007/s00216-021-03602-2.
Bauer M, Wunderlich L, Weinzierl F, Lei Y, Duerkop A, Alshareef HN, et al. Electrochemical multi-analyte point-of-care perspiration sensors using on-chip three-dimensional graphene electrodes. Anal Bioanal Chem. 2021;413:763–77. https://doi.org/10.1007/s00216-020-02939-4.
Chen G, Zheng J, Liu L, Xu L. Application of microfluidics in wearable devices. Small Methods. 2019;3:1–17. https://doi.org/10.1002/smtd.201900688.
Sempionatto JR, Jeerapan I, Krishnan S, Wang J. Wearable chemical sensors: emerging systems for on-body analytical chemistry. Anal Chem. 2019;92:378–96. https://doi.org/10.1021/acs.analchem.9b04668.
Bandodkar AJ, Jeang WJ, Ghaffari R, Rogers JA. Wearable sensors for biochemical sweat analysis. Annu Rev Anal Chem. 2019;12:1–22. https://doi.org/10.1146/annurev-anchem-061318-114910.
Min J, Sempionatto JR, Teymourian H, Wang J, Gao W. Wearable electrochemical biosensors in North America. Biosens Bioelectron. 2021;172. https://doi.org/10.1016/j.bios.2020.112750.
Sempionatto JR, Lin M, Yin L, De la paz E, Pei K, Sonsa-ard T, et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat Biomed Eng. 2021;5:737–48. https://doi.org/10.1038/s41551-021-00685-1.
Koh A, Kang D, Xue Y, Lee S, Pielak RM, Kim J, et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci Transl Med. 2016. https://doi.org/10.1126/scitranslmed.aaf2593
Kim SB, Lee KH, Raj MS, Lee B, Reeder JT, Koo J, et al. Soft, Skin-interfaced microfluidic systems with wireless, battery-free electronics for digital, real-time tracking of sweat loss and electrolyte composition. Small. 2018;14:1–9. https://doi.org/10.1002/smll.201802876.
Bandodkar AJ, Choi J, Lee SP, Jeang WJ, Agyare P, Gutruf P, et al. Soft, Skin-interfaced microfluidic systems with passive galvanic stopwatches for precise chronometric sampling of sweat. Adv Mater. 2019;31:1–9. https://doi.org/10.1002/adma.201902109.
Nyein HYY, Tai LC, Ngo QP, Chao M, Zhang GB, Gao W, et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sensors. 2018;3:944–52. https://doi.org/10.1021/acssensors.7b00961.
Yeo JC, Kenry, Lim CT. Emergence of microfluidic wearable technologies. Lab Chip. 2016;16:4082–90. https://doi.org/10.1039/c6lc00926c.
Choi J, Kang D, Han S, Kim SB, Rogers JA. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv Healthc Mater. 2017;6:1–10. https://doi.org/10.1002/adhm.201601355.
Wang S, Chinnasamy T, Lifson MA, Inci F, Demirci U. Flexible substrate-based devices for point-of-care diagnostics. Trends Biotechnol. 2016;34:909–21. https://doi.org/10.1016/j.tibtech.2016.05.009.
Bandodkar AJ, Gutruf P, Choi J, Lee KH, Sekine Y, Reeder JT, et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci Adv. 2019;5:1–16. https://doi.org/10.1126/sciadv.aav3294.
Heikenfeld J, Jajack A, Feldman B, Granger SW, Gaitonde S, Begtrup G, et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat Biotechnol. 2019;37:407–19. https://doi.org/10.1038/s41587-019-0040-3.
Martín A, Kim J, Kurniawan JF, Sempionatto JR, Moreto JR, Tang G, et al. Epidermal microfluidic electrochemical detection system: enhanced sweat sampling and metabolite detection. ACS Sensors. 2017;2:1860–8. https://doi.org/10.1021/acssensors.7b00729.
Hauke A, Simmers P, Ojha YR, Cameron BD, Ballweg R, Zhang T, et al. Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation. Lab Chip. 2018;18:3750–9. https://doi.org/10.1039/c8lc01082j.
Alizadeh A, Burns A, Lenigk R, Gettings R, Ashe J, Porter A, et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab Chip. 2018;18:2632–41. https://doi.org/10.1039/c8lc00510a.
Vinoth R, Nakagawa T, Mathiyarasu J, Mohan AMV. Fully printed wearable microfluidic devices for high-throughput sweat sampling and multiplexed electrochemical analysis. ACS Sensors. 2021;6:1174–86. https://doi.org/10.1021/acssensors.0c02446.
Sempionatto JR, Martin A, García-Carmona L, Barfidokht A, Kurniawan JF, Moreto JR, et al. Skin-worn soft microfluidic potentiometric detection system. Electroanalysis. 2019;31:239–45. https://doi.org/10.1002/elan.201800414.
Ma B, Chi J, Xu C, Ni Y, Zhao C, Liu H. Wearable capillary microfluidics for continuous perspiration sensing. Talanta. 2020;212. https://doi.org/10.1016/j.talanta.2020.120786.
Ichimura Y, Kuritsubo T, Nagamine K, Nomura A, Shitanda I, Tokito S. A fully screen-printed potentiometric chloride ion sensor employing a hydrogel-based touchpad for simple and non-invasive daily electrolyte analysis. Anal Bioanal Chem. 2021;413:1883–91. https://doi.org/10.1007/s00216-021-03156-3.
Nyein HYY, Bariya M, Tran B, Ahn CH, Brown BJ, Ji W, et al. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat Commun. 2021;12:1–13. https://doi.org/10.1038/s41467-021-22109-z.
Bariya M, Li L, Ghattamaneni R, Ahn CH, Nyein HYY, Tai LC, et al. Glove-based sensors for multimodal monitoring of natural sweat. Sci Adv. 2020;6:1–10. https://doi.org/10.1126/sciadv.abb8308.
Tang W, Yin L, Sempionatto JR, Moon JM, Teymourian H, Wang J. Touch-based stressless cortisol sensing. Adv Mater. 2021;33:1–11. https://doi.org/10.1002/adma.202008465.
Sempionatto JR, Moon JM, Wang J. Touch-based fingertip blood-free reliable glucose monitoring: personalized data processing for predicting blood glucose concentrations. ACS Sensors. 2021;6:1875–83. https://doi.org/10.1021/acssensors.1c00139.
Moon J, Teymourian H, De la Paz E, Sempionatto JR, Mahato K, Sonsa-ard T, et al. Non-invasive sweat-based tracking of L-dopa pharmacokinetic profiles following an oral tablet administration. Angew Chemie. 2021;133:19222–6. https://doi.org/10.1002/ange.202106674.
Yin L, Moon JM, Sempionatto JR, Lin M, Cao M, Trifonov A, et al. A passive perspiration biofuel cell: high energy return on investment. Joule. 2021;5:1888–904. https://doi.org/10.1016/j.joule.2021.06.004.
Su B. Recent progress on fingerprint visualization and analysis by imaging ridge residue components Young Investigators in Analytical and Bioanalytical Science. Anal Bioanal Chem. 2016;408:2781–91. https://doi.org/10.1007/s00216-015-9216-y.
Kuwayama K, Tsujikawa K, Miyaguchi H, Kanamori T, Iwata YT, Inoue H. Time-course measurements of caffeine and its metabolites extracted from fingertips after coffee intake: a preliminary study for the detection of drugs from fingerprints. Anal Bioanal Chem. 2013;405:3945–52. https://doi.org/10.1007/s00216-012-6569-3.
Kim J, Jeerapan I, Imani S, Cho TN, Bandodkar A, Cinti S, et al. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sensors. 2016;1:1011–9. https://doi.org/10.1021/acssensors.6b00356.
Kim J, Sempionatto JR, Imani S, Hartel MC, Barfidokht A, Tang G, et al. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv Sci. 2018;5:1800880. https://doi.org/10.1002/advs.201800880.
Bandodkar AJ, Jia W, Yardımcı C, Wang X, Ramirez J, Wang J. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal Chem. 2015;87:394–8. https://doi.org/10.1021/ac504300n.
Emaminejad S, Gao W, Wu E, Davies ZA, Nyein HYY, Challa S, et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc Natl Acad Sci USA. 2017;114:4625–30. https://doi.org/10.1073/pnas.1701740114.
Nyein HYY, Bariya M, Kivimäki L, Uusitalo S, Liaw TS, Jansson E, et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci Adv. 2019;5. https://doi.org/10.1126/sciadv.aaw9906.
Lin H, Zhao Y, Lin S, Wang B, Yeung C, Cheng X, et al. A rapid and low-cost fabrication and integration scheme to render 3D microfluidic architectures for wearable biofluid sampling, manipulation, and sensing. Lab Chip. 2019;19:2844–53. https://doi.org/10.1039/c9lc00418a.
Peng R, Sonner Z, Hauke A, Wilder E, Kasting J, Gaillard T, et al. A new oil/membrane approach for integrated sweat sampling and sensing: sample volumes reduced from μL’s to nL’s and reduction of analyte contamination from skin. Lab Chip. 2016;16:4415–23. https://doi.org/10.1039/c6lc01013j.
Kim J, Campbell AS, de Ávila BEF, Wang J. Wearable biosensors for healthcare monitoring. Nat Biotechnol. 2019;37:389–406. https://doi.org/10.1038/s41587-019-0045-y.
Kim J, Campbell AS, Wang J. Wearable non-invasive epidermal glucose sensors: a review. Talanta. 2018;177:163–70. https://doi.org/10.1016/j.talanta.2017.08.077.
Vettoretti M, Cappon G, Acciaroli G, Facchinetti A, Sparacino G. Continuous glucose monitoring: current use in diabetes management and possible future applications. J Diabetes Sci Technol. 2018;12:1064–71. https://doi.org/10.1177/1932296818774078.
Karpova EV, Shcherbacheva EV, Galushin AA, Vokhmyanina DV, Karyakina EE, Karyakin AA. Noninvasive diabetes monitoring through continuous analysis of sweat using flow-through glucose biosensor. Anal Chem. 2019;91:3778–83. https://doi.org/10.1021/acs.analchem.8b05928.
Choi J, Xue Y, Xia W, Ray TR, Reeder JT, Bandodkar AJ, et al. Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab Chip. 2017;17:2572–80. https://doi.org/10.1039/c7lc00525c.
Sonner Z, Wilder E, Heikenfeld J, Kasting G, Beyette F, Swaile D, et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics. 2015;9. https://doi.org/10.1063/1.4921039.
Sonner Z, Wilder E, Gaillard T, Kasting G, Heikenfeld J. Integrated sudomotor axon reflex sweat stimulation for continuous sweat analyte analysis with individuals at rest. Lab Chip. 2017;17:2550–60. https://doi.org/10.1039/c7lc00364a.
Funding
This work was supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense (grant number: HDTRA 1–16-1–0013) and the UCSD Center of Wearable Sensors (CWS). J. R. S. received fellowship from CNPq (grant number: 216981/2014–0), G. B. received fellowship from the Scientific and Technological Research Council of Turkey (TUBITAK 2219). N. F. A. received fellowship from Capes/PDSE—Finance Code 001 (Program 194 – Process—88881.186873/2018–01). E.D.l.P. received support from a UC MEXUS–CONACYT collaborative fellowship (2017–2022).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
Human studies were conducted in strict compliance following a protocol approved by the Institutional Review Board (IRB) at the University of California, San Diego. All participants provided written informed consent prior to each study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Promising Early-Career (Bio)Analytical Researchers with guest editors Antje J. Baeumner, María C. Moreno-Bondi, Sabine Szunerits, and Qiuquan Wang.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bolat, G., De la Paz, E., Azeredo, N.F. et al. Wearable soft electrochemical microfluidic device integrated with iontophoresis for sweat biosensing. Anal Bioanal Chem 414, 5411–5421 (2022). https://doi.org/10.1007/s00216-021-03865-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03865-9