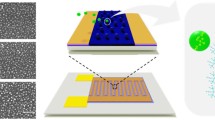
Abstract
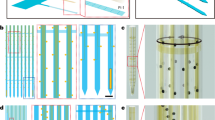
An SU-8 probe with an array of nine, individually addressable gold microband electrodes (100 μm long, 4 μm wide, separated by 4-μm gaps) was photolithographically fabricated and characterized for detection of low concentrations of chemicals in confined spaces and in vivo studies of biological tissues. The probe’s shank (6 mm long, 100 μm wide, 100 μm thick) is flexible, but exhibits sufficient sharpness and rigidity to be inserted into soft tissue. Laser micromachining was used to define probe geometry by spatially revealing the underlying sacrificial aluminum layer, which was then etched to free the probes from a silicon wafer. Perfusion with fluorescent nanobeads showed that, like a carbon fiber electrode, the probe produced no noticeable damage when inserted into rat brain, in contrast to damage from an inserted microdialysis probe. The individual addressability of the electrodes allows single and multiple electrode activation. Redox cycling is possible, where adjacent electrodes serve as generators (that oxidize or reduce molecules) and collectors (that do the opposite) to amplify signals of small concentrations without background subtraction. Information about electrochemical mechanisms and kinetics may also be obtained. Detection limits for potassium ferricyanide in potassium chloride electrolyte of 2.19, 1.25, and 2.08 μM and for dopamine in artificial cerebral spinal fluid of 1.94, 1.08, and 5.66 μM for generators alone and for generators and collectors during redox cycling, respectively, were obtained.
Graphical abstract










Similar content being viewed by others
References
Xu X, Zhang S, Chen H, Kong J. Integration of electrochemistry in micro-total analysis systems for biochemical assays: recent developments. Talanta. 2009;80(1):8–18. https://doi.org/10.1016/j.talanta.2009.06.039.
Niwa O. Electroanalytical chemistry with carbon film electrodes and micro and nano-structured carbon film-based electrodes. Bull Chem Soc Jpn. 2005;78(4):555–71. https://doi.org/10.1246/bcsj.78.555.
Lowinsohn D, Peres HEM, Kosminsky L, Paixão TRLC, Ferreira TL, Ramirez-Fernandez FJ, et al. Design and fabrication of a microelectrode array for iodate quantification in small sample volumes. Sensors Actuators B Chem. 2006;113(1):80–7. https://doi.org/10.1016/j.snb.2005.02.024.
Hu M, Fritsch I. Redox cycling behavior of individual and binary mixtures of catecholamines at gold microband electrode arrays. J Anal Chem. 2015;87(4):2029–32.
Aggarwal A. Studies toward the development of a microelectrode array for detection of dopamine through redox cycling. Dissertation, University of Arkansas; 2011.
Niwa O. Electroanalysis with interdigitated array microelectrodes. Electroanalysis. 1995;7(7):606–13. https://doi.org/10.1002/elan.1140070702.
Bard AJ, Crayston JA, Kittlesen GP, Varco Shea T, Wrighton MS. Digital simulation of the measured electrochemical response of reversible redox couples at microelectrode arrays: consequences arising from closely spaced ultramicroelectrodes. Anal Chem. 1986;58(11):2321–31. https://doi.org/10.1021/ac00124a045.
Oleinick A, Zhu F, Yan J, Mao B, Svir I, Amatore C. Theoretical investigation of generator-collector microwell arrays for improving electroanalytical selectivity: application to selective dopamine detection in the presence of ascorbic acid. Chem Phys Chem. 2013;14(9):1887–98. https://doi.org/10.1002/cphc.201300134.
Seymour JP, Wu F, Wise KD, Yoon E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst Nanoeng. 2017;3(1):16066. https://doi.org/10.1038/micronano.2016.66.
Jaquins-Gerstl A, Michael AC. Comparison of the brain penetration injury associated with microdialysis and voltammetry. J Neurosci Methods. 2009;183(2):127–35. https://doi.org/10.1016/j.jneumeth.2009.06.023.
Hong G, Lieber CM. Novel electrode technologies for neural recordings. Nat Neurosci. 2019;20(6):330–45. https://doi.org/10.1038/s41583-019-0140-6.
Dengler AK, McCarty GS. Microfabricated microelectrode sensor for measuring background and slowly changing dopamine concentrations. J Electroanal Chem. 2013;693:28–33. https://doi.org/10.1016/j.jelechem.2013.01.022.
Zachek MK, Park J, Takmakov P, Wightman RM, McCarty GS. Microfabricated FSCV-compatible microelectrode array for real-time monitoring of heterogeneous dopamine release. Analyst. 2010;135(7):1556–63. https://doi.org/10.1039/c0an00114g.
Roberts JG, Sombers LA. Fast-scan cyclic voltammetry: chemical sensing in the brain and beyond. Anal Chem. 2018;90(1):490–504. https://doi.org/10.1021/acs.analchem.7b04732.
Rodeberg NT, Sandberg SG, Johnson JA, Phillips PEM, Wightman RM. Hitchhiker’s guide to voltammetry: acute and chronic electrodes for in vivo fast-scan cyclic voltammetry. ACS Chem Neurosci. 2017;8(2):221–34. https://doi.org/10.1021/acschemneuro.6b00393.
Hunsberger HC, Setti SE, Heslin RT, Quintero JE, Gerhardt GA, Reed MN. Using enzyme-based biosensors to measure tonic and phasic glutamate in Alzheimer’s mouse models. JoVE. 2017;123:e55418.
Burmeister JJ, Pomerleau F, Huettl P, Gash CR, Werner CE, Bruno JP, et al. Ceramic-based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosens Bioelectron. 2008;23(9):1382–9. https://doi.org/10.1016/j.bios.2007.12.013.
Burmeister JJ, Price DA, Pomerleau F, Huettl P, Quintero JE, Gerhardt GA. Challenges of simultaneous measurements of brain extracellular GABA and glutamate in vivo using enzyme-coated microelectrode arrays. 2020; (1872-678X (Electronic)).
Zachek MK, Takmakov P, Park J, Wightman RM, McCarty GS. Simultaneous monitoring of dopamine concentration at spatially different brain locations in vivo. Biosens Bioelectron. 2010;25(5):1179–85. https://doi.org/10.1016/j.bios.2009.10.008.
Ngernsutivorakul T, White TS, Kennedy RT. Microfabricated probes for studying brain chemistry. Chem Phys Chem. 2018;19(10):1128.
Xi Y. Interdigitated array electrode microprobe: design, fabrication and characterization: University of Arkansas; 2005.
Cao Q, Puthongkham P, Venton BJ. Review: new insights into optimizing chemical and 3D surface structures of carbon electrodes for neurotransmitter detection. Anal Methods-UK. 2019;11(3):247–61. https://doi.org/10.1039/C8AY02472C.
Atcherley CW, Laude ND, Parent KL, Heien ML. Fast-scan controlled-adsorption voltammetry for the quantification of absolute concentrations and adsorption dynamics. Langmuir. 2013;29(48):14885–92.
Taylor IM, Patel NA, Freedman NC, Castagnola E, Cui XT. Direct in vivo electrochemical detection of resting dopamine using poly (3, 4-ethylenedioxythiophene)/carbon nanotube functionalized microelectrodes. Anal Chem. 2019;91(20):12917–27.
Pathirathna P, Balla RJ, Amemiya S. Nanogap-based electrochemical measurements at double-carbon-fiber ultramicroelectrodes. Anal Chem. 2018;90(20):11746–50. https://doi.org/10.1021/acs.analchem.8b02987.
Hu M, Fritsch I. Application of electrochemical redox cycling: toward differentiation of dopamine and norepinephrine. Anal Chem. 2016;88(11):5574–8.
HajjHassan M, Chodavarapu V, Musallam S. NeuroMEMS: neural probe microtechnologies. Sensors. 2008;8(10):6704–26. https://doi.org/10.3390/s8106704.
Sreenivas G, Ang SS, Fritsch I, Brown WD, Gerhardt GA, Woodward DJ. Fabrication and characterization of sputtered-carbon microelectrode arrays. Anal Chem. 1996;68(11):1858–64.
Metallo C, White RD, Trimmer BA. Flexible parylene-based microelectrode arrays for high resolution EMG recordings in freely moving small animals. J Neurosci Methods. 2011;195(2):176–84.
Zhuolin Xiang S-CY, Xue N, Sun T. Ultra-thin flexible polyimide neural probe embedded in dissolvable maltose-coated microneedle. J Micromech Microeng. 2014;24:11–20.
Huang S-H, Lin S-P, Chen J-JJ. In vitro and in vivo characterization of SU-8 flexible neuroprobe: From mechanical properties to electrophysiological recording. Sensors Actuators A Phys. 2014;216:257–65. https://doi.org/10.1016/j.sna.2014.06.005.
Takeuchi S, Ziegler D, Yoshida Y, Mabuchi K, Suzuki T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip. 2005;5(5):519–23.
Keekeun Lee JH. Ryan Clement. Biocompatible benzyclobutene (BCB)-based neural implants with micro-fluidic channel. Biosens Bioelectron. 2004;20:404–7.
A. H. A. Malavazi JABG, R. J. M. Covolan, R. R. Panepucci. Design and microfabrication methodology of Su-8 based neural probes XXIV Congresso Brasileiro de Engenharia Biomédica. 2014:2850–3.
Nemani KV, Moodie KL, Brennick JB, Su A, Gimi B. In vitro and in vivo evaluation of SU-8 biocompatibility. Mater Sci Eng. 2013;33(7):4453–9. https://doi.org/10.1016/j.msec.2013.07.001.
Read TL, Cobb SJ, Macpherson JV. An sp2 patterned boron doped diamond electrode for the simultaneous detection of dissolved oxygen and pH. Sensors. 2019;4(3):756–63. https://doi.org/10.1021/acssensors.9b00137.
Li A, Chan SH, Nguyen N-T. A laser-micromachined polymeric membraneless fuel cell. J Micromech Microeng. 2007;17(6):1107–13. https://doi.org/10.1088/0960-1317/17/6/002.
Ghantasala MK, Hayes JP, Harvey EC, Sood DK. Patterning, electroplating and removal of SU-8 moulds by excimer laser micromachining. J Micromech Microeng. 2001;11(2):133–9. https://doi.org/10.1088/0960-1317/11/2/308.
Green RA, Ordonez JS, Schuettler M, Poole-Warren LA, Lovell NH, Suaning GJ. Cytotoxicity of implantable microelectrode arrays produced by laser micromachining. Biomaterials. 2010;31(5):886–93. https://doi.org/10.1016/j.biomaterials.2009.09.099.
Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52(5):1655–8. https://doi.org/10.1111/j.1471-4159.1989.tb09224.x.
Matarèse BFE, Feyen PLC, Falco A, Benfenati F, Lugli P, deMello JC. Use of SU8 as a stable and biocompatible adhesion layer for gold bioelectrodes. Sci Rep-UK. 2018;8(1):5560. https://doi.org/10.1038/s41598-018-21755-6.
Nesbitt KM, Jaquins-Gerstl A, Skoda EM, Wipf P, Michael AC. Pharmacological mitigation of tissue damage during brain microdialysis. Anal Chem. 2013;85(17):8173–9. https://doi.org/10.1021/ac401201x.
Hajj-Hassan M, Fayad R, Berro S, Chodavarapu VP, Musallam S. Implantation of elongated porous silicon neural probe array in rat cortex. Preprints. 2018.
Mitch Taylor I, Jaquins-Gerstl A, Sesack SR, Michael AC. Domain-dependent effects of DAT inhibition in the rat dorsal striatum. J Neurochem. 2012;122(2):283–94.
Aggarwal A, Hu M, Fritsch I. Detection of dopamine in the presence of excess ascorbic acid at physiological concentrations through redox cycling at an unmodified microelectrode array. Anal Bioanal Chem. 2013;405. https://doi.org/10.1007/s00216-013-6738-z.
Venton BJ, Cao Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst. 2020;145(4):1158–68. https://doi.org/10.1039/C9AN01586H.
Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. 2nd ed. New York: Wiley; 2001.
Konopka SJ, McDuffie B. Diffusion coefficients of ferri- and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal Chem. 1970;42(14):1741–6. https://doi.org/10.1021/ac50160a042.
Le Drogoff B, El Khakani MA, Silva PRM, Chaker M, Vijh AK. Effect of the microelectrode geometry on the diffusion behavior and the electroanalytical performance of hg-electroplated iridium microelectrode arrays intended for the detection of heavy metal traces. Electroanal. 2001;13(18):1491–6. https://doi.org/10.1002/1521-4109(200112)13:18<1491::AID-ELAN1491>3.0.CO;2-Z.
Aoki K, Morita M, Niwa O, Tabei H. Quantitative analysis of reversible diffusion-controlled currents of redox soluble species at interdigitated array electrodes under steady-state conditions. J Electroanal Chem. 1988;256(2):269–82.
Niwa O, Morita M, Tabei H. Fabrication and characteristics of vertically separated interdigitated array electrodes. J Electroanal Chem. 1989;267(1):291–7. https://doi.org/10.1016/0022-0728(89)80257-8.
Han D, Zaino Iii LP, Fu K, Bohn PW. Redox cycling in nanopore-confined recessed dual-ring electrode arrays. J Phys Chem C. 2016;120(37):20634–41.
Niwa O, Morita M, Tabei H. Highly sensitive and selective voltammetric detection of dopamine with vertically separated interdigitated array electrodes. Electroanalysis. 1991;3(3):163–8. https://doi.org/10.1002/elan.1140030305.
Rice ME, Gerhardt GA, Hierl PM, Nagy G, Adams RN. Diffusion coefficients of neurotransmitters and their metabolites in brain extracellular fluid space. Neuroscience. 1985;15(3):891–902. https://doi.org/10.1016/0306-4522(85)90087-9.
Wouters K, Puers R. Diffusing and swelling in SU-8: insight in material properties and processing. J Micromech Microeng. 2010;20(9):095013. https://doi.org/10.1088/0960-1317/20/9/095013.
Gu H, Varner EL, Groskreutz SR, Michael AC, Weber SG. In vivo monitoring of dopamine by microdialysis with 1 min temporal resolution using online capillary liquid chromatography with electrochemical detection. Anal Chem. 2015;87(12):6088–94.
Yang H, Thompson AB, McIntosh BJ, Altieri SC, Andrews AM. Physiologically relevant changes in serotonin resolved by fast microdialysis. ACS Chem Neurosci. 2013;4(5):790–8.
Robbins EM, Jaquins-Gerstl A, Fine DF, Leong CL, Dixon CE, Wagner AK, et al. Extended (10-day) real-time monitoring by dexamethasone-enhanced microdialysis in the injured rat cortex. ACS Chem Neurosci. 2019;10(8):3521–31. https://doi.org/10.1021/acschemneuro.9b00145.
Roberts JG, Lugo-Morales LZ, Loziuk PL, Sombers LA. Real-time chemical measurements of dopamine release in the brain. Methods Mol Biol. 2013;964:275–94. https://doi.org/10.1007/978-1-62703-251-3_16.
Acknowledgements
MLM is grateful for a Fulbright Dissertation Research Award. We thank Dr. Ben J. Jones for designing the graphical abstract. We acknowledge Errol Porter for consultation on microelectrode fabrication and the High-Density Electronics Center for use of microfabrication facilities. Scanning electron microscopy images were obtained in the Arkansas Nano & Biomaterials Characterization Facility at the University of Arkansas. We are grateful to Dr. Jonathan Moldenhauer and Professor David Paul at the University of Arkansas for designing and providing the circuit board for mounting the edge connector.
Source of biological material
Male Sprague−Dawley rats were obtained from Charles River, Raleigh, NC.
Funding
Research was supported partially through the National Institutes of Health (R21NS086107) and the University of Pittsburgh Center for Biological Imaging (1S10RR028478-01), the Royal Society for an Industry Fellowship (J.V.M., INF/R1/180026), and the Centre for Doctoral Training in Diamond Science and Technology (EP/ L015315/1) with the Defense Science and Technology Laboratory (Dstl) (S.J.C.), the University of Arkansas’s Women’s Giving Circle, the National Science Foundation (CMI-1808286), and the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The University of Pittsburgh’s Institutional Animal Care and Use Committee reviewed and approved all procedures involving animals.
Statement on animal welfare
All animal procedures were in compliance with USDA laboratory animal use regulations. Additionally, all procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh, the location of the laboratory where all experiments involving animals took place.
Conflict of interest
The authors declare no competing interests.
Additional information
Published in the topical collection Electrochemistry for Neurochemical Analysis with guest editors Ashley E. Ross and Alexander G. Zestos.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 250 kb)
Rights and permissions
About this article
Cite this article
Lotfi Marchoubeh, M., Cobb, S.J., Abrego Tello, M. et al. Miniaturized probe on polymer SU-8 with array of individually addressable microelectrodes for electrochemical analysis in neural and other biological tissues. Anal Bioanal Chem 413, 6777–6791 (2021). https://doi.org/10.1007/s00216-021-03327-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03327-2