Abstract
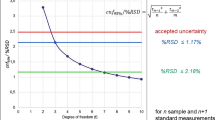
The drugs used for treatment during chemotherapy are manufactured individually for each patient in specialised pharmacies. Thorough quality control to confirm the identity of the delivered active pharmaceutical ingredient and the final concentration of the prepared application solution is not standardized yet except for optical or gravimetric testing. However, solution stability problems, counterfeit drugs, and erroneous or deliberate underdosage may occur and negatively influence the quality of the product and could cause severe health risks for the patient. To take a step towards analytical quality control, an on-site analytical instrument using Raman and UV absorption spectroscopy was employed and the results were compared to high-performance liquid chromatography coupled to diode array detection. Within the scope of the technology evaluation, the uncertainty of measurement was determined for the analysis of the five frequently used cytostatic drugs 5-fluorouracil, cyclophosphamide, gemcitabine, irinotecan and paclitaxel. The Raman/UV technique (2.0–3.2% uncertainty of measurement; level of confidence: 95%) achieves a combined uncertainty of measurement comparable to HPLC-DAD (1.7–3.2% uncertainty of measurement; level of confidence: 95%) for the substances 5-fluorouracil, cyclophosphamide and gemcitabine. However, the uncertainty of measurement for the substances irinotecan and paclitaxel is three times higher when the Raman/UV technique is used. This is due to the fact that the Raman/UV technique analyses the undiluted sample; therefore, the sample has a higher viscosity and tendency to foam. Out of 136 patient-specific preparations analysed within this study, 96% had a deviation of less than 10% from the target content.
Graphical abstract





Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredients
- HPLC-DAD:
-
High-performance liquid chromatography coupled with diode array detection
- Raman/UV:
-
Raman spectroscopy combined with ultraviolet detection
- CP:
-
Cyclophosphamide
- IF:
-
Ifosfamide
- Ph. Eur.:
-
European Pharmacopoeia
- USP:
-
United States Pharmacopeia
- Ph. Helv.:
-
Pharmacopoea Helvetica
- 5-FU:
-
5-Fluorouracil
- Gem:
-
Gemcitabine
- Irino:
-
Irinotecan
- Pac:
-
Paclitaxel
- FA:
-
Formic acid
- UV:
-
Ultraviolet
- MAGS:
-
Ministry of Labour, Health and Social Affairs of the state of North Rhine-Westphalia in Germany
References
Muller T. Typical medication errors in oncology: analysis and prevention strategies. Onkologie. 2003;26(6):539–44. https://doi.org/10.1159/000074148.
Sendra-Garcia A, Martinez-Gomez MA, Albert-Mari A, Jimenez-Torres NV, Climente-Marti M. Quantitative and qualitative control of antineoplastic preparations: Gravimetry versus HPLC. J Oncol Pharm Pract. 2019;25(5):1204–16. https://doi.org/10.1177/1078155219834999.
Benizri F, Dalifard B, Zemmour C, Henriquet M, Fougereau E, Le Franc B. DrugCam (R)-an intelligent video camera system to make safe cytotoxic drug preparations. Int J Pharm. 2016;502(1–2):198–207. https://doi.org/10.1016/j.ijpharm.2016.02.028.
Bazin C, Cassard B, Caudron E, Prognon P, Havard L. Comparative analysis of methods for real-time analytical control of chemotherapies preparations. Int J Pharm. 2015;494(1):329–36. https://doi.org/10.1016/j.ijpharm.2015.08.041.
Lagarce F. Centrally prepared cytotoxic drugs: what is the purpose of their quality control? Pharm Technol Hosp Pharm. 2017;2(1):29–33. https://doi.org/10.1515/pthp-2017-0006.
Bouligand J, Paci A, Mercier L, Vassal G, Bourget P. High-performance thin-layer chromatography with a derivatization procedure, a suitable method for the identification and the quantitation of busulfan in various pharmaceutical products. J Pharmaceut Biomed. 2004;34(3):525–30. https://doi.org/10.1016/S0731-7085(03)00630-7.
Paci A, Mercier L, Bourget P. Identification and quantitation of antineoplastic compounds in chemotherapeutic infusion bags by use of HPTLC: application to the vinca-alkaloids. J Pharmaceut Biomed. 2003;30(5):1603–10. https://doi.org/10.1016/S0731-7085(02)00541-1.
Delmas A, Gordien JB, Bernadou JM, Roudaut M, Gresser A, Malki L, et al. Quantitative and qualitative control of cytotoxic preparations by HPLC-UV in a centralized parenteral preparations unit. J Pharmaceut Biomed. 2009;49(5):1213–20. https://doi.org/10.1016/j.jpba.2009.03.007.
Jaccoulet E, Schweitzer-Chaput A, Toussaint B, Prognon P, Caudron E. Simple and ultra-fast recognition and quantitation of compounded monoclonal antibodies: application to flow injection analysis combined to UV spectroscopy and matching method. Talanta. 2018;187:279–86. https://doi.org/10.1016/j.talanta.2018.05.042.
Jaccoulet E, Smadja C, Prognon P, Taverna M. Capillary electrophoresis for rapid identification of monoclonal antibodies for routine application in hospital. Electrophoresis. 2015;36(17):2050–6. https://doi.org/10.1002/elps.201400603.
Nardella F, Beck M, Collart-Dutilleul P, Becker G, Boulanger C, Perello L, et al. A UV-Raman spectrometry method for quality control of anticancer preparations: results after 18 months of implementation in hospital pharmacy. Int J Pharm. 2016;499(1–2):343–50. https://doi.org/10.1016/j.ijpharm.2016.01.002.
Chouquet T, Benoit G, Morand K. Analytical control of pediatric chemotherapy preparations with a UV-Raman automaton: results after 18 months of implementation and development of a suitable method for low volume preparations. Pharm Technol Hosp Pharm. 2017;2(3):117–29. https://doi.org/10.1515/pthp-2017-0021.
Le LMM, Caudron E, Baillet-Guffroy A, Eyeleigh L. Non-invasive quantification of 5-fluorouracil and gemcitabine in aqueous matrix by direct measurement through glass vials using near-infrared spectroscopy. Talanta. 2014;119:361–6. https://doi.org/10.1016/j.talanta.2013.10.060.
Le L, Berge M, Tfayli A, Guffroy AB, Prognon P, Dowek A, et al. Quantification of gemcitabine intravenous drugs by direct measurement in chemotherapy plastic bags using a handheld Raman spectrometer. Talanta. 2019;196:376–80. https://doi.org/10.1016/j.talanta.2018.11.062.
Deutscher Apotheker Verlag. European Pharmacopoeia, 9th Ed.; 2018.
Schmiedel. DA-VDR. USP 42 - NF 37 The United States Pharmacopeia and National Formulary 2019: Main Edition Plus Supplements 1 and 2; 2018.
Swissmedic. Pharmacopoea Helvetica, 11th Ed.; 2019.
Busch A. Ergebnisse von Apothekenkontrollen Aktenzeichen IV B 5 - G.0611; 2019.
Council Directive. Council Directive 90/394/EEC of 28 June 1990 on the protection of workers from the risks related to exposure to carcinogens at work (Sixth individual Directive within the meaning of Article 16 (1) of Directive 89/391/EEC); 1990.
Working Group 1 of the Joint Committee for Guides in Metrology. Evaluation of measurement data — Guide to the expression of uncertainty in measurement; 2008.
Mandel J. The statistical analysis of experimental data. Washington: Wiley; 1964.
Pharmacopoea Europaea. Test for extractable volume of parenteral preparations; 2018.
Cohen MR, Smetzer JL. Understanding and managing intravenous container overfill; potential dose confusion. Hosp Pharm. 2014;49(3):221–6. https://doi.org/10.1310/hpj4903-221.
Thiessen JJ. A review of the oncology under-dosing incident. 2013. URL: http://www.health.gov.on.ca/en/public/programs/cancer/drugsupply/docs/report_thiessen_oncology_under-dosing.pdf Last accessed: 07.09.2020.
DIN EN ISO/IEC 17025:2018 General requirements for the competence of testing and calibration laboratories. 2017.
Dziopa F, Galy G, Bauler S, Vincent B, Crochon S, Tall ML, et al. A quantitative and qualitative method to control chemotherapeutic preparations by Fourier transform infrared–ultraviolet spectrophotometry. J Oncol Pharm Pract. 2013;19(2):121–9. https://doi.org/10.1177/1078155212457963.
Acknowledgements
We want to thank the company B&W Tek for providing the i-QCRx and Sara Seiffert for her kind support and fruitful discussion.
Funding
We would like to thank the Federal Ministry for Economic Affairs and Energy for funding the INNO-KOM project “Sensitive method for the detection of airborne proteins” (49VF170039).
Author information
Authors and Affiliations
Contributions
Lars M. H. Reinders: Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing (original draft), writing (review and editing), visualization, project administration, funding acquisition. Martin D. Klassen: Conceptualization, methodology, writing (review and editing), supervision, funding acquisition. Claudia vom Eyser: Writing (review and editing), project administration. Thorsten Teutenberg: Conceptualization, writing (review and editing), supervision, funding acquisition. Martin Jaeger: Writing (review and editing), supervision. Torsten C. Schmidt: Writing (review and editing), supervision. Jochen Tuerk: Conceptualization, writing (review and editing), supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 452 kb)
Rights and permissions
About this article
Cite this article
Reinders, L.M.H., Klassen, M.D., vom Eyser, C. et al. Quality control of cytostatic drug preparations—comparison of workflow and performance of Raman/UV and high-performance liquid chromatography coupled with diode array detection (HPLC-DAD). Anal Bioanal Chem 413, 2587–2596 (2021). https://doi.org/10.1007/s00216-021-03223-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03223-9