Abstract
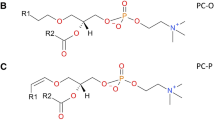
Phosphatidylethanolamines (PEs) are targets of non-enzymatic glycation, a chemical process that occurs between glucose and primary amine-containing biomolecules. As the early-stage non-enzymatic glycation products of PE, Amadori-PEs are implicated in the pathogenesis of various diseases. However, only a few Amadori-PE molecular species have been identified so far; a comprehensive profiling of these glycated PE species is needed to establish their roles in disease pathology. Herein, based on our previous work using liquid chromatography-coupled neutral loss scanning and product ion scanning tandem mass spectrometry (LC-NLS-MS and LC-PIS-MS) in tandem, we extend identification of Amadori-PE to the low-abundance species, which is facilitated by using plasma lipids glycated in vitro. The confidence of identification is improved by high-resolution tandem mass spectrometry and chromatographic retention time regression. A LC-coupled multiple reaction monitoring mass spectrometry (LC-MRM-MS) assay is further developed for more sensitive quantitation of the Amadori compound-modified lipids. Using synthesized stable isotope-labeled Amadori lipids as internal standards, levels of 142 Amadori-PEs and 33 Amadori-LysoPEs are determined in the NIST human plasma standard reference material. These values may serve as an important reference for future investigations of Amadori-modified lipids in human diseases.





Similar content being viewed by others
References
Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51(11):3299–305.
Zhang Q, Ames JM, Smith RD, Baynes JW, Metz TO. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J Proteome Res. 2009;8(2):754–69.
Miyazawa T, Nakagawa K, Shimasaki S, Nagai R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42(4):1163–70.
Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A. 1993;90(14):6434–8.
Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A. 1994;91(20):9441–5.
Sookwong P, Nakagawa K, Fujita I, Shoji N, Miyazawa T. Amadori-glycated phosphatidylethanolamine, a potential marker for hyperglycemia, in streptozotocin-induced diabetic rats. Lipids. 2011;46(10):943–52.
Eitsuka T, Nakagawa K, Ono Y, Tatewaki N, Nishida H, Kurata T, et al. Amadori-glycated phosphatidylethanolamine up-regulates telomerase activity in PANC-1 human pancreatic carcinoma cells. FEBS Lett. 2012;586(16):2542–7.
Oak J-H, Nakagawa K, Oikawa S, Miyazawa T. Amadori-glycated phosphatidylethanolamine induces angiogenic differentiations in cultured human umbilical vein endothelial cells. FEBS Lett. 2003;555(2):419–23.
Nakagawa K, Oak JH, Higuchi O, Tsuzuki T, Oikawa S, Otani H, et al. Ion-trap tandem mass spectrometric analysis of Amadori-glycated phosphatidylethanolamine in human plasma with or without diabetes. J Lipid Res. 2005;46(11):2514–24.
Kodate A, Otoki Y, Shimizu N, Ito J, Kato S, Umetsu N, et al. Development of quantitation method for glycated aminophospholipids at the molecular species level in powdered milk and powdered buttermilk. Sci Rep. 2018;8(1):8729.
Nakagawa K, Ibusuki D, Suzuki Y, Yamashita S, Higuchi O, Oikawa S, et al. Ion-trap tandem mass spectrometric analysis of squalene monohydroperoxide isomers in sunlight-exposed human skin. J Lipid Res. 2007;48(12):2779–87.
He X, Chen GY, Zhang Q. Comprehensive identification of Amadori compound-modified phosphatidylethanolamines in human plasma. Chem Res Toxicol. 2019;32(7):1449–57.
He X, Zhang Q. Synthesis, purification, and mass spectrometric characterization of stable isotope-labeled Amadori-glycated phospholipids. ACS Omega. 2018;3(11):15725–33.
Bowden JA, Heckert A, Ulmer CZ, Jones CM, Koelmel JP, Abdullah L, et al. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950-metabolites in frozen human plasma. J Lipid Res. 2017;58(12):2275–88.
Ovcacikova M, Lisa M, Cifkova E, Holcapek M. Retention behavior of lipids in reversed-phase ultrahigh-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A. 2016;1450:76–85.
Vu N, Narvaez-Rivas M, Chen GY, Rewers MJ, Zhang Q. Accurate mass and retention time library of serum lipids for type 1 diabetes research. Anal Bioanal Chem. 2019;411(23):5937–49.
Miyazawa T, Ibusuki D, Yamashita S, Nakagawa K. Analysis of amadori-glycated phosphatidylethanolamine in the plasma of healthy subjects and diabetic patients by liquid chromatography-tandem mass spectrometry. Ann N Y Acad Sci. 2008;1126:291–4.
Shoji N, Nakagawa K, Asai A, Fujita I, Hashiura A, Nakajima Y, et al. LC-MS/MS analysis of carboxymethylated and carboxyethylated phosphatidylethanolamines in human erythrocytes and blood plasma. J Lipid Res. 2010;51(8):2445–53.
Miyazawa T, Oak JH, Nakagawa K. Tandem mass spectrometry analysis of Amadori-glycated phosphatidylethanolamine in human plasma. Ann N Y Acad Sci. 2005;1043:280–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
De-identified, commercial human plasma was used in this work. Research conducted with unidentified samples is not considered human subjects research and is not regulated by the Federal Policy for the Protection of Human Subjects (45 CFR Part 46).
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, X., Li, Z. & Zhang, Q. A UPLC-MRM-MS method for comprehensive profiling of Amadori compound-modified phosphatidylethanolamines in human plasma. Anal Bioanal Chem 413, 431–443 (2021). https://doi.org/10.1007/s00216-020-03012-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-03012-w