Abstract
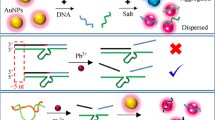
Detection of lead(II) (Pb2+) ions in water is important for the protection of human health and environment. The growing demand for onsite detection still faces challenges for sensitive and easy-to-use methods. In this work, a novel surface plasmon resonance (SPR) biosensor based on GR-5 DNAzyme and gold nanoparticles (AuNPs) was developed. Thiolated DNAzyme was immobilized on the gold surface of the sensor chip followed by anchoring the substrate-functionalized AuNPs through the DNAzyme-substrate hybridization. The coupling between the localized surface plasmon (LSP) of AuNPs and the surface plasmon polaritons (SPP) on the gold sensor surface was used to improve the sensitivity. The substrate cleavage in the presence of Pb2+ ions was catalyzed by DNAzyme, leading to the removal of AuNPs and the diminished LSP-SPP coupling. The optimal detection limit was 80 pM for the sensor fabricated with 1 μM DNAzyme, corresponding to two or three orders of magnitude lower than the toxicity levels of Pb2+ in drinking water defined by WHO and USEPA. By tuning the surface coverage of DNAzyme, the sensitivity and dynamic range could be controlled. This sensor also featured high selectivity to Pb2+ ions and simple detection procedure. Successful detection of Pb2+ ions in groundwater indicates that this method has the prospect in the onsite detection of Pb2+ ions in water. Given the variety of AuNPs and metal-specific DNAzymes, this detection strategy would lead to the development of more sensitive and versatile heavy metal sensors.

Graphical abstract






Similar content being viewed by others
References
Yang QQ, Li ZY, Lu XN, Duan QN, Huang L, Bi J. A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Sci Total Environ. 2018;642:690–700.
Khoshbin Z, Housaindokht MR, Verdian A, Bozorgmehr MR. Simultaneous detection and determination of mercury (II) and lead (II) ions through the achievement of novel functional nucleic acid-based biosensors. Biosens Bioelectron. 2018;116:130–47.
Babu SH, Kumar KS, Suvardhan K, Kiran K, Rekha D, Krishnaiah L, et al. Preconcentration technique for the determination of trace elements in natural water samples by ICP-AES. Environ Monit Assess. 2007;128(1–3):241–9.
Cocherie A, Robert M. Direct measurement of lead isotope ratios in low concentration environmental samples by MC-ICP-MS and multi-ion counting. Chem Geol. 2007;243(1–2):90–104.
Mortada WI, Kenawy IMM, Abdelghany AM, Ismal AM, Donia AF, Nabieh KA. Determination of Cu2+ Zn2+ and Pb2+ in biological and food samples by FAAS after preconcentration with hydroxyapatite nanorods originated from eggshell. Mater Sci Eng C. 2015;52:288–96.
Liu S, Sun JS, Huo JZ, Duan XC, Liu Y. Room-temperature chelate vapor generation of lead using ammonium O,O-diethyl dithiophosphate as a chelating reagent and determination by atomic fluorescence spectrometry in environmental water samples. Anal Sci. 2019;35(5):499–504.
Sun Q, Wang J, Tang M, Huang L, Zhang Z, Liu C, et al. A new electrochemical system based on a flow-field shaped solid electrode and 3D-printed thin-layer flow cell: detection of Pb2+ ions by continuous flow accumulation square-wave anodic stripping voltammetry. Anal Chem. 2017;89(9):5024–9.
Malik LA, Bashir A, Qureashi A, Pandith AH. Detection and removal of heavy metal ions: a review. Environ Chem Lett. 2019;17(4):1495–521.
Zhao G, Liu G. A portable electrochemical system for the on-site detection of heavy metals in farmland soil based on electrochemical sensors. IEEE Sensors J. 2018;18(14):5645–55.
Lu W, Lin C, Yang J, Wang X, Yao B, Wang M. A DNAzyme assay coupled with effective magnetic separation and rolling circle amplification for detection of lead cations with a smartphone camera. Anal Bioanal Chem. 2019;411(21):5383–91.
Kim H, Jang G, Yoon Y. Specific heavy metal/metalloid sensors: current state and perspectives. Appl Microbiol Biotechnol. 2020;104(3):907–14.
Liang G, Man Y, Li A, Jin X, Liu X, Pan L. DNAzyme-based biosensor for detection of lead ion: a review. Microchem J. 2017;131:145–53.
Cuenoud B, Szostak JW. A DNA metalloenzyme with DNA-ligase activity. Nature. 1995;375(6532):611–4.
Tan Y, Qiu J, Cui M, Wei X, Zhao M, Qiu B, et al. An immobilization free DNAzyme based electrochemical biosensor for lead determination. Analyst. 2016;141(3):1121–6.
Liang H, Xie S, Cui L, Wu C, Zhang X. Designing a biostable L-DNAzyme for lead(II) ion detection in practical samples. Anal Methods. 2016;8(39):7260–4.
Cheng Y, Huang Y, Lei J, Zhang L, Ju H. Design and biosensing of Mg2+-dependent DNAzyme-triggered ratiometric electrochemiluminescence. Anal Chem. 2014;86(10):5158–63.
Zhou Y, Tang L, Zeng G, Zhang C, Zhang Y, Xie X. Current progress in biosensors for heavy metal ions based on DNAzymes/DNA molecules functionalized nanostructures: a review. Sens Actuators B Chem. 2016;223:280–94.
Lan T, Furuya K, Lu Y. A highly selective lead sensor based on a classic lead DNAzyme. Chem Commun. 2010;46(22):3896–8.
Lee JH, Wang Z, Liu J, Lu Y. Highly sensitive and selective colorimetric sensors for uranyl (UO22+): development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems. J Am Chem Soc. 2008;130(43):14217–26.
Brown AK, Liu J, He Y, Lu Y. Biochemical characterization of a uranyl ion-specific DNAzyme. Chembiochem. 2009;10(3):486–92.
Fu T, Ren S, Gong L, Meng H, Cui L, Kong R-M, et al. A label-free DNAzyme fluorescence biosensor for amplified detection of Pb2+-based on cleavage-induced G-quadruplex formation. Talanta. 2016;147:302–6.
Liu MY, Lou XH, Du J, Guan M, Wang J, Ding XF, et al. DNAzyme-based fluorescent microarray for highly selective and sensitive detection of lead (II). Analyst. 2012;137(1):70–2.
Wang H-B, Wang L, Huang K-J, Xu S-P, Wang H-Q, Wang L-L, et al. A highly sensitive and selective biosensing strategy for the detection of Pb2+ ions based on GR-5 DNAzyme functionalized AuNPs. New J Chem. 2013;37(8):2557–63.
Yun W, Cai D, Jiang J, Zhao P, Huang Y, Sang G. Enzyme-free and label-free ultra-sensitive colorimetric detection of Pb2+ using molecular beacon and DNAzyme based amplification strategy. Biosens Bioelectron. 2016;80:187–93.
Yang X, Xu J, Tang X, Liu H, Tian D. A novel electrochemical DNAzyme sensor for the amplified detection of Pb2+ ions. Chem Commun. 2010;46(18):3107–9.
Xiao Y, Rowe AA, Plaxco KW. Electrochemical detection of parts-per-billion lead via an electrode-bound DNAzyme assembly. J Am Chem Soc. 2007;129(2):262–3.
Ji R, Niu W, Chen S, Xu W, Ji X, Yuan L, et al. Target-inspired Pb2+−dependent DNAzyme for ultrasensitive electrochemical sensor based on MoS2-AuPt nanocomposites and hemin/G-quadruplex DNAzyme as signal amplifier. Biosens Bioelectron. 2019;144:111560.
Tian A, Liu Y, Gao J. Sensitive SERS detection of lead ions via DNAzyme based quadratic signal amplification. Talanta. 2017;171:185–9.
Wang Y, Irudayaraj J. A SERS DNAzyme biosensor for lead ion detection. Chem Commun. 2011;47(15):4394–6.
Teh HB, Li H, Li SFY. Highly sensitive and selective detection of Pb2+ ions using a novel and simple DNAzyme-based quartz crystal microbalance with dissipation biosensor. Analyst. 2014;139(20):5170–5.
Daniya WMEMM, Saleviter S, Fen YW. Development of surface plasmon resonance spectroscopy for metal ion detection. Sens Mater. 2018;30(9):2023–38.
Fen YW, Yunus WMM. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: a review. Sens Rev. 2013;33(4):305–14.
Wu H, Li H, Chua FZH, Li SFY. Rapid detection of melamine based on immunoassay using portable surface plasmon resonance biosensor. Sens Actuators B Chem. 2013;178:541–6.
Guner H, Ozgur E, Kokturk G, Celik M, Esen E, Topal AE, et al. A smartphone based surface plasmon resonance imaging (SPRi) platform for on-site biodetection. Sens Actuators B Chem. 2017;239:571–7.
Alwahib AAA, Kamil YM, Abu Bakar MH, Noor ASM, Yaacob MH, Lim HN, et al. Reduced graphene oxide/maghemite nanocomposite for detection of lead ions in water using surface plasmon resonance. IEEE Photon J. 2018;10(6):1–10.
Lyon LA, Musick MD, Natan MJ. Colloidal Au-enhanced surface plasmon resonance immunosensing. Anal Chem. 1998;70(24):5177–83.
Zeng S, Yong K-T, Roy I, Dinh X-Q, Yu X, Luan F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics. 2011;6(3):491–506.
Fathi F, Rashidi M-R, Omidi Y. Ultra-sensitive detection by metal nanoparticles-mediated enhanced SPR biosensors. Talanta. 2019;192:118–27.
Wang L, Li T, Du Y, Chen C, Li L, Zhou M, et al. Au NPs-enhanced surface plasmon resonance for sensitive detection of mercury (II) ions. Biosens Bioelectron. 2010;25(12):2622–6.
Mustafa DE, Yang T, Xuan Z, Chen S, Tu H, Zhang A. Surface plasmon coupling effect of gold nanoparticles with different shape and size on conventional surface plasmon resonance signal. Plasmonics. 2010;5(3):221–31.
Guo L, Jackman JA, Yang H-H, Chen P, Cho N-J, Kim D-H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today. 2015;10(2):213–39.
Petrovykh DY, Kimura-Suda H, Whitman LJ, Tarlov MJ. Quantitative analysis and characterization of DNA immobilized on gold. J Am Chem Soc. 2003;125(17):5219–26.
Jia Y, Li F. Studies of functional nucleic acids modified light addressable potentiometric sensors: X-ray photoelectron spectroscopy, biochemical assay, and simulation. Anal Chem. 2018;90(8):5153–61.
Kawasaki M, Sato T, Tanaka T, Takao K. Rapid self-assembly of alkanethiol monolayers on sputter-grown Au(111). Langmuir. 2000;16(4):1719–28.
Petrovykh DY, Kimura-Suda H, Tarlov MJ, Whitman LJ. Quantitative characterization of DNA films by X-ray photoelectron spectroscopy. Langmuir. 2004;20(2):429–40.
He L, Musick MD, Nicewarner SR, Salinas FG, Benkovic SJ, Natan MJ, et al. Colloidal Au-enhanced surface plasmon resonance for ultrasensitive detection of DNA hybridization. J Am Chem Soc. 2000;122(38):9071–7.
Besselink GAJ, Kooyman RPH, van Os P, Engbers GHM, Schasfoort RBM. Signal amplification on planar and gel-type sensor surfaces in surface plasmon resonance-based detection of prostate-specific antigen. Anal Biochem. 2004;333(1):165–73.
Mock JJ, Hill RT, Degiron A, Zauscher S, Chilkoti A, Smith DR. Distance-dependent plasmon resonant coupling between a gold nanoparticle and gold film. Nano Lett. 2008;8(8):2245–52.
Frasconi M, Tortolini C, Botre F, Mazzei F. Multifunctional au nanoparticle dendrimer-based surface plasmon resonance biosensor and its application for improved insulin detection. Anal Chem. 2010;82(17):7335–42.
Hinman SS, McKeating KS, Cheng Q. Surface plasmon resonance: material and interface design for universal accessibility. Anal Chem. 2018;90(1):19–39.
Qiu G, Ng SP, Liang X, Ding N, Chen X, Wu C-ML. Label-free LSPR detection of trace lead(II) ions in drinking water by synthetic poly(mPD-co-ASA) nanoparticles on gold nanoislands. Anal Chem. 2017;89(3):1985–93.
Wang JJ, Morabito K, Tang JX, Tripathi A. Microfluidic platform for isolating nucleic acid targets using sequence specific hybridization. Biomicrofluidics. 2013;7:044107.
Jiang KR, Huang JL, Chen CC, Su HJ, Wu JC. Effect of co-axially hybridized gene targets on hybridization efficiency of microarrayed DNA probes. J Taiwan Inst Chem Eng. 2011;42(1):5–12.
Demers LM, Mirkin CA, Mucic RC, Reynolds RA, Letsinger RL, Elghanian R, et al. A fluorescence-based method for determining the surface coverage and hybridization efficiency of thiol-capped oligonucleotides bound to gold thin films and nanoparticles. Anal Chem. 2000;72(22):5535–41.
Fu C, Xu W, Wang H, Ding H, Liang L, Cong M, et al. DNAzyme-based plasmonic nanomachine for ultrasensitive selective surface-enhanced Raman scattering detection of lead ions via a particle-on-a-film hot spot construction. Anal Chem. 2014;86(23):11494–7.
Zhang Y, Xiao S, Li H, Liu H, Pang P, Wang H, et al. A Pb2+-ion electrochemical biosensor based on single-stranded DNAzyme catalytic beacon. Sens Actuators B Chem. 2016;222:1083–9.
Ma R-N, Wang LL, Zhang M, Jia L-P, Zhang W, Shang L, et al. A novel one-step triggered “signal-on/off” electrochemical sensing platform for lead based on the dual-signal ratiometric output and electrode-bound DNAzyme assembly. Sens Actuators B Chem. 2018;257:678–84.
Fen YW, Yunus WMM, Yusof NA. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix 4 arene-tetrakis in chitosan thin film as an active layer. Sens Actuators B Chem. 2012;171:287–93.
Liao X, Luo J, Wu J, Fan T, Yao Y, Gao F, et al. A sensitive DNAzyme-based electrochemical sensor for Pb2+ detection with platinum nanoparticles decorated TiO2/alpha-Fe2O3 nanocomposite as signal labels. J Electroanal Chem. 2018;829:129–37.
Wu J, Wei S, Lu Y, Ren N, Bian X, Zhang J. Ultrasensitive DNAzyme-based electrochemical biosensor for Pb2+ based on FcHT-mediated biocatalytic amplification. Int J Electrochem Sci. 2018;13(10):9630–41.
Yu Z, Li N, Hu X, Dong Y, Lin Y, Cai H, et al. Highly efficient electrochemical detection of lead ion using metal-organic framework and graphene as platform based on DNAzyme. Synth Met. 2019;254:164–71.
Jing JY, Zhu Q, Dai ZX, Li SY, Wang Q, Zhao WM. Sensing self-referenced fiber optic long-range surface plasmon resonance sensor based on electronic coupling between surface plasmon polaritons. Appl Opt. 2019;58(23):6329–34.
Shalabney A, Abdulhalim I. Sensitivity-enhancement methods for surface plasmon sensors. Laser Photonics Rev. 2011;5(4):571–606.
Kim S, Lee HJ. Gold nanostar enhanced surface plasmon resonance detection of an antibiotic at attomolar concentrations via an aptamer-antibody sandwich assay. Anal Chem. 2017;89(12):6624–30.
Jang HR, Wark AW, Baek SH, Chung BH, Lee HJ. Ultrasensitive and ultrawide range detection of a cardiac biomarker on a surface plasmon resonance platform. Anal Chem. 2014;86(1):814–9.
Hutter E, Fendler JH, Roy D. Surface plasmon resonance studies of gold and silver nanoparticles linked to gold and silver substrates by 2-aminoethanethiol and 1,6-hexanedithiol. J Phys Chem B. 2001;105(45):11159–68.
Funding
This research was supported by the National Key R&D Program of China (2018YFC1902903) and Shenzhen Science and Technology Innovation Committee (JSGG20170822164024506 and KJYY20171012103638606).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1164 kb).
Rights and permissions
About this article
Cite this article
Wu, H., Wang, S., Li, S.F.Y. et al. A label-free lead(II) ion sensor based on surface plasmon resonance and DNAzyme-gold nanoparticle conjugates. Anal Bioanal Chem 412, 7525–7533 (2020). https://doi.org/10.1007/s00216-020-02887-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02887-z