Abstract
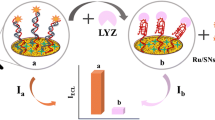
Lysozyme (LYZ) sensors have attracted increased attention because rapid and sensitive detection of LYZ is highly desirable for various diseases associated with humans. In this research, we designed l-cysteine-protected ultra small photoluminescent (PL) copper nanoclusters (CuNCs) conjugated with β-cyclodextrin (β-CD) for rapid detection of LYZ in human serum samples at room temperature. The proposed β-CD-CuNCs exhibited excellent water solubility, ultrafine size, good dispersion, bright photoluminescence, and good photostability. The β-CD-CuNCs exhibit an excitation and emission maximum at 370 nm and 492 nm, respectively, with an absolute quantum yield (QY) of 54.6%. β-CD-CuNCs showed a fluorescence lifetime of 12.7 ns. The addition of LYZ would result in PL quenching from β-CD-CuNCs. The lowest detectable LYZ concentration was 50 nM for the naked eye and the limit of detection (LOD) and limit of quantification (LOQ) were 0.36 nM and 1.21 nM, respectively, by emission measurement observed in the LYZ concentration range from 30 to 100 nM. It is important to note that the PL β-CD-CuNC strategy possessed great selectivity toward LYZ relative to other biomolecules. The proposed nanosensor was efficiently applied to determine the LYZ level in human serum sample average recoveries from 96.15 to 104.05% and relative standard deviation (RSD) values lower than 3.0%. Moreover, the proposed sensing system showed many advantages, including high speed, high sensitivity, high selectivity, low cost, and simple preparation.









Similar content being viewed by others
References
Jolles P. Lysozymes: model enzymes in biochemistry and biology. Exp Suppl. 1996;75:449. https://doi.org/10.1007/978-3-0348-9225-4.
Swaminathan R, Ravi VK, Kumar S, Kumar MVS, Chandra N Lysozyme: a model protein for amyloid research. Adv Protein Chem Struct Biol. 2011;84:63–111. https://doi.org/10.1016/B978-0-12-386483-3.00003-3
Callewaert L, Michiels CW. Lysozymes in the animal kingdom. J Biosci. 2010;35:127–60. https://doi.org/10.1007/s12038-010-0015-5.
Yan L, Shen S, Yun J, Yao K. Isolation of lysozyme from chicken egg white using polyacrylamide-based cation-exchange cryogel. Chinese J Chem Eng. 2011;19:876–80. https://doi.org/10.1016/S1004-9541(11)60068-2.
González DC, Savariar EN, Thayumanavan S. Fluorescence patterns from supramolecular polymer assembly and disassembly for sensing metallo- and nonmetalloproteins. J Am Chem Soc. 2009;131:7708–16. https://doi.org/10.1021/ja900579g.
Levinson SS, Elin RJ, Yam L. Light chain proteinuria and lysozymuria in a patient with acute monocytic leukemia. Clin Chem. 2002;48:1131–2. https://doi.org/10.1093/clinchem/48.7.1131.
Cai Z, Chen G, Huang X, Ma M. Determination of lysozyme at the nanogram level in chicken egg white using resonance Rayleigh-scattering method with Cd-doped ZnSe quantum dots as probe. Sensors Actuators B Chem. 2011;157:368–73. https://doi.org/10.1016/j.snb.2011.04.058.
Lie SQ, Zou HY, Chang Y, Huang CZ. Tuning of the near-infrared localized surface plasmon resonance of Cu2-xSe nanoparticles with lysozyme-induced selective aggregation. RSC Adv. 2014;4:55094–9. https://doi.org/10.1039/c4ra05828c.
Mörsky P. Turbidimetric determination of lysozyme with Micrococcus lysodeikticus cells: reexamination of reaction conditions. Anal Biochem. 1983;128:77–85. https://doi.org/10.1016/0003-2697(83)90347-0.
Liu DY, Xin YY, He XW, Yin XB. A sensitive, non-damaging electrochemiluminescent aptasensor via a low potential approach at DNA-modified gold electrodes. Analyst. 2011;136:479–85. https://doi.org/10.1039/c0an00607f.
Pellegrino L, Tirelli A. A sensitive HPLC method to detect hen’s egg white lysozyme in milk and dairy products. Int Dairy J. 2000;10:435–42. https://doi.org/10.1016/S0958-6946(00)00065-0.
Zhang L, Wang E. Metal nanoclusters: new fluorescent probes for sensors and bioimaging. Nano Today. 2014;9:132–57. https://doi.org/10.1016/j.nantod.2014.02.010.
Li J, Zhu JJ, Xu K. Fluorescent metal nanoclusters: from synthesis to applications. TrAC - Trends Anal Chem. 2014;58:90–8. https://doi.org/10.1016/j.trac.2014.02.011.
Wilcoxon JP, Abrams BL. Synthesis, structure and properties of metal nanoclusters. Chem Soc Rev. 2006;35:1162–94. https://doi.org/10.1039/b517312b.
Shang L, Dong S, Nienhaus GU. Ultra-small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today. 2011;6:401–18. https://doi.org/10.1016/j.nantod.2011.06.004.
Lu Y, Chen W. Sub-nanometre sized metal clusters: from synthetic challenges to the unique property discoveries. Chem Soc Rev. 2012;41:3594–623. https://doi.org/10.1039/c2cs15325d.
Jin R. Atomically precise metal nanoclusters: stable sizes and optical properties. Nanoscale. 2015;7:1549–65. https://doi.org/10.1039/c4nr05794e.
Anandhakumar S, Rajaram R, Mathiyarasu J. Unusual seedless approach to gold nanoparticle synthesis: application to selective rapid naked eye detection of mercury(ii). Analyst. 2013;139:3356–9. https://doi.org/10.1039/c4an00480a.
Bian P, Xing L, Liu Z, Ma Z. Functionalized-tryptophan stabilized fluorescent Ag nanoclusters: synthesis and its application as Hg2+ ions sensor. Sensors Actuators B Chem. 2014;203:252–7. https://doi.org/10.1016/j.snb.2014.06.133.
Stelzhammer V, Ozcan S, Michael G, Steeb H, Hodes GE, Guest C, et al. Diagnostics in Neuropsychiatry. 2015:88–93. https://doi.org/10.1016/j.dineu.2015.08.001.
Jia X, Yang X, Li J, Li D, Wang E. Stable Cu nanoclusters: from an aggregation-induced emission mechanism to biosensing and catalytic applications. Chem Commun. 2014;50:237–9. https://doi.org/10.1039/c3cc47771a.
Yang X, Feng Y, Zhu S, Luo Y, Zhuo Y, Dou Y. One-step synthesis and applications of fluorescent Cu nanoclusters stabilized by l-cysteine in aqueous solution. Anal Chim Acta. 2014;847:49–54. https://doi.org/10.1016/j.aca.2014.07.019.
Barthel MJ, Angeloni I, Petrelli A, Avellini T, Scarpellini A, Bertoni G, et al. Synthesis of highly fluorescent copper clusters using living polymer chains as combined reducing agents and ligands. ACS Nano. 2015;9:11886–97. https://doi.org/10.1021/acsnano.5b04270.
Del Valle EMM. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–46. https://doi.org/10.1016/S0032-9592(03)00258-9.
Sonaimuthu M, Balakrishnan SB, Kuppu SV, Veerakanellore GB, Thambusamy S. Spectral and proton transfer behavior of 1,4-dihydroxylanthraquinone in aqueous and confined media; molecular modelling strategy. J Mol Liq. 2018;259:186–98. https://doi.org/10.1016/j.molliq.2018.03.042.
Mohandoss S, Stalin T. Photochemical and computational studies of inclusion complexes between β-cyclodextrin and 1,2-dihydroxyanthraquinones. Photochem Photobiol Sci. 2017;16:476–88. https://doi.org/10.1039/c6pp00285d.
Mohandoss S, Sivakamavalli J, Vaseeharan B, Stalin T. Host-guest molecular recognition based fluorescence on-off-on chemosensor for nanomolar level detection of Cu2+ and Cr2O72- ions: application in XNOR logic gate and human lung cancer living cell imaging. Sens Actuators, B Chem. 2016;234:300–15. https://doi.org/10.1016/j.snb.2016.04.148.
Watanabe S, Sato S, Ohtsuka K, Takenaka S. Electrochemical DNA analysis with a supramolecular assembly of naphthalene diimide, ferrocene, and β-cyclodextrin. Anal Chem. 2011;83:7290–6. https://doi.org/10.1021/ac200989c.
Chen X, Cheng X, Justin Gooding J. Detection of trace nitroaromatic isomers using indium tin oxide electrodes modified using β-cyclodextrin and silver nanoparticles. Anal Chem. 2012;84:8557–63. https://doi.org/10.1021/ac3014675.
Zhao X, Liu X, Lu M. β-Cyclodextrin-capped palladium nanoparticle-catalyzed ligand-free Suzuki and Heck couplings in low-melting β-cyclodextrin/NMU mixtures. Appl Organomet Chem. 2014;28:635–40. https://doi.org/10.1002/aoc.3173.
Putta C, Sharavath V, Sarkar S, Ghosh S. Palladium nanoparticles on β-cyclodextrin functionalised graphene nanosheets: a supramolecular based heterogeneous catalyst for C-C coupling reactions under green reaction conditions. RSC Adv. 2015;5:6652–60. https://doi.org/10.1039/c4ra14323j.
Chen S, Zhang J, Gan N, Hu F, Li T, Cao Y, et al. An on-site immunosensor for ractopamine based on a personal glucose meter and using magnetic β-cyclodextrin-coated nanoparticles for enrichment, and an invertase-labeled nanogold probe for signal amplification. Microchim Acta. 2014;182:815–22. https://doi.org/10.1007/s00604-014-1392-5.
Wang M, Wu H, Chi Y, Chen G. Synthesis of Au13(glutathionato)8@β-cyclodextrin nanoclusters and their use as a fluorescent probe for silver ions. Microchim Acta. 2014;181:1573–80. https://doi.org/10.1007/s00604-014-1253-2.
Zhong Y, He Y, Ge Y, Song G. β-Cyclodextrin protected Cu nanoclusters as a novel fluorescence sensor for graphene oxide in environmental water samples. Luminescence. 2017;32:596–601. https://doi.org/10.1002/bio.3226.
Lakkakula JR, Divakaran D, Thakur M, Kumawat MK, Srivastava R. Cyclodextrin-stabilized gold nanoclusters for bioimaging and selective label-free intracellular sensing of Co2+ ions. Sens Actuators, B Chem. 2018;262:270–81. https://doi.org/10.1016/j.snb.2018.01.219.
Halawa MI, Wu F, Fereja TH, Lou B, Xu G. One-pot green synthesis of supramolecular Β-cyclodextrin functionalized gold nanoclusters and their application for highly selective and sensitive fluorescent detection of dopamine. Sens Actuators, B Chem. 2018;254:1017–24. https://doi.org/10.1016/j.snb.2017.07.201.
Ban R, Abdel-Halim ES, Zhang J, Zhu JJ. β-Cyclodextrin functionalised gold nanoclusters as luminescence probes for the ultrasensitive detection of dopamine. Analyst. 2015;140:1046–53. https://doi.org/10.1039/c4an02161d.
Wang X, Gao W, Xu W, Xu S. Fluorescent Ag nanoclusters templated by carboxymethyl-β-cyclodextrin (CM-β-CD) and their in vitro antimicrobial activity. Mater Sci Eng C. 2013;33:656–62. https://doi.org/10.1016/j.msec.2012.10.012.
Zhao Y, Huang Y, Zhu H, Zhu Q, Xia Y. Three-in-one: sensing, self-assembly, and cascade catalysis of cyclodextrin modified gold nanoparticles. J Am Chem Soc. 2016;138:16645–54. https://doi.org/10.1021/jacs.6b07590.
Tang C, Qian Z, Huang Y, Xu J, Ao H, Zhao M, et al. A fluorometric assay for alkaline phosphatase activity based on β-cyclodextrin-modified carbon quantum dots through host-guest recognition. Biosens Bioelectron. 2016;83:274–80. https://doi.org/10.1016/j.bios.2016.04.047.
Behbehani GR, Barzegar L. Thermal study of lysozyme binding with β-cyclodextrin. Appl Mech Mater. 2012;110–116:1966–9. https://doi.org/10.4028/www.scientific.net/AMM.110-116.1966.
Yamamoto T, Fukui N, Hori A, Matsui Y. Circular dichroism and fluorescence spectroscopy studies of the effect of cyclodextrins on the thermal stability of chicken egg white lysozyme in aqueous solution. J Mol Struct. 2006;782:60–6. https://doi.org/10.1016/j.molstruc.2005.01.024.
Paquin F, Rivnay J, Salleo A, Stingelin N, Silva C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J Mater Chem C. 2015;3:10715–22. https://doi.org/10.1039/b000000x.
Senra JD, Malta LFB, Michel RC, Cordeiro Y, Simão RA, Simas ABC, et al. Hydrophilic cyclodextrin protected Pd nanoclusters: insights into their size control and host-guest behavior. J Mater Chem. 2011;21:13516–23. https://doi.org/10.1039/c1jm11962a.
Sharma N, Baldi A. Exploring versatile applications of cyclodextrins: an overview. Drug Deliv. 2016;23:739–57. https://doi.org/10.3109/10717544.2014.938839.
Rigo A, Corazza A, Luisa Di Paolo M, Rossetto M, Ugolini R, Scarpa M. Interaction of copper with cysteine: stability of cuprous complexes and catalytic role of cupric ions in anaerobic thiol oxidation. J Inorg Biochem. 2004;98:1495–501. https://doi.org/10.1016/j.jinorgbio.2004.06.008.
Eli I (1981) Laussnne -Printed in the Netherlands 85. 12:85–101.
Mott D, Galkowski J, Wang L, Luo J, Zhong CJ. Synthesis of size-controlled and shaped copper nanoparticles. Langmuir. 2007;23:5740–5. https://doi.org/10.1021/la0635092.
Goswami N, Yao Q, Luo Z, Li J, Chen T, Xie J. Luminescent metal nanoclusters with aggregation-induced emission. J Phys Chem Lett. 2016;7:962–75. https://doi.org/10.1021/acs.jpclett.5b02765.
Wang Z, Zhang CC, Gao J, Wang Q. Copper clusters-based luminescence assay for tetracycline and cellular imaging studies. J Lumin. 2017;190:115–22. https://doi.org/10.1016/j.jlumin.2017.05.038.
Luo Y, Miao H, Yang X. Glutathione-stabilized Cu nanoclusters as fluorescent probes for sensing pH and vitamin B1. Talanta. 2015;144:488–95. https://doi.org/10.1016/j.talanta.2015.07.001.
Wang LL, Wang QL, Xu XY, Li JZ, Gao L, Bin, et al. Energy transfer from Bi3+ to Eu3+ triggers exceptional long-wavelength excitation band in ZnWO4:Bi3+, Eu3+ phosphors. J Mater Chem C. 2013;1:8033–40. https://doi.org/10.1039/c3tc31160k.
Hu X, Liu T, Zhuang Y, Wang W, Li Y, Fan W, et al. Recent advances in the analytical applications of copper nanoclusters. TrAC - Trends Anal Chem. 2016;77:66–75. https://doi.org/10.1016/j.trac.2015.12.013.
Cui M, Song G, Wang C, Song Q. Synthesis of cysteine-functionalized water-soluble luminescent copper nanoclusters and their application to the determination of chromium(VI). Microchim Acta. 2015;182:1371–7. https://doi.org/10.1007/s00604-015-1458-z.
Zhou T, Xu W, Yao Q, Zhao T, Chen X. Highly fluorescent copper nanoclusters as a probe for the determination of pH. Methods Appl Fluoresc. 2015;3:7. https://doi.org/10.1088/2050-6120/3/4/044002.
Yuan X, Luo Z, Zhang Q, Zhang X, Zheng Y, Lee JY, et al. Synthesis of highly fluorescent metal (Ag, Au, Pt, and Cu) nanoclusters by electrostatically induced reversible phase transfer. ACS Nano. 2011;5:8800–8. https://doi.org/10.1021/nn202860s.
Tao Y, Li M, Ren J, Qu X. Metal nanoclusters: novel probes for diagnostic and therapeutic applications. Chem Soc Rev. 2015;44:8636–63. https://doi.org/10.1039/c5cs00607d.
Wang Z, Chen B, Susha AS, Wang W, Reckmeier CJ, Chen R, et al. All-copper nanocluster based down-conversion white light-emitting devices. Adv Sci. 2016;3. https://doi.org/10.1002/advs.201600182.
Wang Z, Susha AS, Chen B, Reckmeier C, Tomanec O, Zboril R, et al. Poly(vinylpyrrolidone) supported copper nanoclusters: glutathione enhanced blue photoluminescence for application in phosphor converted light emitting devices. Nanoscale. 2016;8:7197–202. https://doi.org/10.1039/c6nr00806b.
Cao H, Chen Z, Zheng H, Huang Y. Copper nanoclusters as a highly sensitive and selective fluorescence sensor for ferric ions in serum and living cells by imaging. Biosens Bioelectron. 2014;62:189–95. https://doi.org/10.1016/j.bios.2014.06.049.
Boschen JS, Lee J, Windus TL, Evans JW, Liu DJ. Size dependence of S-bonding on (111) facets of Cu nanoclusters. J Phys Chem C. 2016;120:10268–74. https://doi.org/10.1021/acs.jpcc.6b00829.
Krylova V, Andruleviçius M. Optical, XPS and XRD studies of semiconducting copper sulfide layers on a polyamide film. Int J Photoenergy. 2009, 2009. https://doi.org/10.1155/2009/304308.
Abarghoei S, Fakhri N, Borghei YS, Hosseini M, Ganjali MR. A colorimetric paper sensor for citrate as biomarker for early stage detection of prostate cancer based on peroxidase-like activity of cysteine-capped gold nanoclusters. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2019;210:251–9. https://doi.org/10.1016/j.saa.2018.11.026.
Li W, Gao Z, Su R, Qi W, Wang L, He Z. Scissor-based fluorescent detection of pepsin using lysozyme-stabilized Au nanoclusters. Anal Methods. 2014;6:6789–95. https://doi.org/10.1039/c4ay00983e.
Higashi T, Hirayama F, Yamashita S, Misumi S, Arima H, Uekama K. Slow-release system of pegylated lysozyme utilizing formation of polypseudorotaxanes with cyclodextrins. Int J Pharm. 2009;374:26–32. https://doi.org/10.1016/j.ijpharm.2009.02.017.
Yamamoto T, Kobayashi T, Yoshikiyo K, Matsui Y, Takahashi T, Aso Y. A 1H NMR spectroscopic study on the tryptophan residues of lysozyme included by glucosyl-β-cyclodextrin. J Mol Struct. 2009;920:264–9. https://doi.org/10.1016/j.molstruc.2008.10.058.
Chen YM, Yu CJ, Cheng TL, Tseng WL. Colorimetric detection of lysozyme based on electrostatic interaction with human serum albumin-modified gold nanoparticles. Langmuir. 2008;24:3654–60. https://doi.org/10.1021/la7034642.
Wang C, Shu S, Yao Y, Song Q. A fluorescent biosensor of lysozyme-stabilized copper nanoclusters for the selective detection of glucose. RSC Adv. 2015;5:101599–606. https://doi.org/10.1039/c5ra19421k.
Desai A, Lee C, Sharma L, Sharma A. Lysozyme refolding with cyclodextrins: structure-activity relationship. Biochimie. 2006;88:1435–45. https://doi.org/10.1016/j.biochi.2006.05.008.
Borghei YS, Hosseini M, Ganjali MR. Fluorescence based turn-on strategy for determination of microRNA-155 using DNA-templated copper nanoclusters. Microchim Acta. 2017;184:2671–7. https://doi.org/10.1007/s00604-017-2272-6.
Subramanian P, Lesniewski A, Kaminska I, Vlandas A, Vasilescu A, Niedziolka-Jonsson J, et al. Lysozyme detection on aptamer functionalized graphene-coated SPR interfaces. Biosens Bioelectron. 2013;50:239–43. https://doi.org/10.1016/j.bios.2013.06.026.
Mihai I, Vezeanu A, Polonschii C, Albu C, Radu GL, Vasilescu A. Label-free detection of lysozyme in wines using an aptamer based biosensor and SPR detection. Sensors Actuators B Chem. 2015;206:198–204. https://doi.org/10.1016/j.snb.2014.09.050.
Chen Z, Guo J. A reagentless signal-off architecture for electrochemical aptasensor for the detection of lysozyme. Electrochim Acta. 2013;111:916–20. https://doi.org/10.1016/j.electacta.2013.08.116.
Liu S, Na W, Pang S, Shi F, Su X. A label-free fluorescence detection strategy for lysozyme assay using CuInS2 quantum dots. Analyst. 2014;139:3048–54. https://doi.org/10.1039/c4an00160e.
Shrivas K, Nirmalkar N, Deb MK, Dewangan K, Nirmalkar J, Kumar S. Application of functionalized silver nanoparticles as a biochemical sensor for selective detection of lysozyme protein in milk sample. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2019;213:127–33. https://doi.org/10.1016/j.saa.2019.01.039.
Zuo L, Qin G, Lan Y, Wei Y, Dong C. A turn-on phosphorescence aptasensor for ultrasensitive detection of lysozyme in humoral samples. Sensors Actuators B Chem. 2019;289:100–5. https://doi.org/10.1016/j.snb.2019.03.088.
Saberi Z, Rezaei B, Rezaei P, Ensafi AA. Design a fluorometric aptasensor based on CoOOH nanosheets and carbon dots for simultaneous detection of lysozyme and adenosine triphosphate. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2020;233. https://doi.org/10.1016/j.saa.2020.118197.
Chen S, Huang Z, Jia Q. Electrostatically confined in-situ preparation of stable glutathione-capped copper nanoclusters for fluorescence detection of lysozyme. Sensors Actuators B Chem. 2020;319:128305. https://doi.org/10.1016/j.snb.2020.128305.
Li S, Gao Z, Shao N. Non-covalent conjugation of CdTe QDs with lysozyme binding DNA for fluorescent sensing of lysozyme in complex biological sample. Talanta. 2014;129:86–92. https://doi.org/10.1016/j.talanta.2014.04.062.
Halawa MI, Lai J, Xu G. Gold nanoclusters: synthetic strategies and recent advances in fluorescent sensing. Mater Today Nano. 2018;3:9–27. https://doi.org/10.1016/j.mtnano.2018.11.001.
Ocaña C, Hayat A, Mishra R, Vasilescu A, Del Valle M, Marty JL. A novel electrochemical aptamer-antibody sandwich assay for lysozyme detection. Analyst. 2015;140:4148–53. https://doi.org/10.1039/c5an00243e.
Cai Z, Yu H, Ma M. Determination of lysozyme at the nanogram level in food sample using resonance Rayleigh-scattering method with Au nanoparticles as probe. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2011;78:1266–71. https://doi.org/10.1016/j.saa.2010.12.074.
Vasilescu A, Gáspár S, Gheorghiu M, David S, Dinca V, Peteu S, et al. Surface plasmon resonance based sensing of lysozyme in serum on Micrococcus lysodeikticus-modified graphene oxide surfaces. Biosens Bioelectron. 2017;89:525–31. https://doi.org/10.1016/j.bios.2016.03.040.
Liao Y-H, Brown MB, Martin GP. Turbidimetric and HPLC assays for the determination of formulated lysozyme activity. J Pharm Pharmacol. 2001;53:549–54. https://doi.org/10.1211/0022357011775668.
Funding
This work was financially supported by the Ministry of Science and Technology of Taiwan (MOST) (MOST 107-2113-M-110-011-MY3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human participants and/or animals
The human blood sample was collected from our lab donors. Human serum sample experiments were conducted after the informed consent of the donors and the approval of the ethics committee of National Sun Yat-Sen University 2018–2019.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 523 kb)
Rights and permissions
About this article
Cite this article
Sonaimuthu, M., Nerthigan, Y., Swaminathan, N. et al. Photoluminescent hydrophilic cyclodextrin-stabilized cysteine-protected copper nanoclusters for detecting lysozyme. Anal Bioanal Chem 412, 7141–7154 (2020). https://doi.org/10.1007/s00216-020-02847-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02847-7