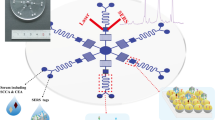
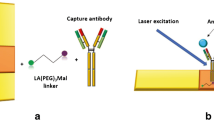
Abstract
Ultrasensitive detection of specific biomarkers in clinical serum is helpful for early diagnosis of cervical cancer. In this paper, a surface-enhanced Raman scattering (SERS)-based immunoassay was developed for the simultaneous determination of squamous cell carcinoma antigen (SCCA) and osteopontin (OPN) in cervical cancer serum. Au-Ag nanoshuttles (Au-AgNSs) as SERS tags and hydrophobic filter paper-based Au nanoflowers (AuNFs) as capture substrate were constructed into a sandwich structure which served as an ultrasensitive SERS-based immunoassay platform. Finite difference time domain simulation confirmed that the electromagnetic field coupled between the AuNFs had a prominent SERS signal enhancement effect, which improved the detection sensitivity. SERS mapping showed that hexadecenyl succinic anhydride hydrophobic treatment could prevent the analyte from being quickly absorbed by the filter paper and increase the retention time to be more evenly distributed on the filter paper substrate. The immunoassay platform was verified to have good selectivity and reproducibility. With this method, the detection limits of SCCA and OPN in human serum were as low as 8.628 pg/mL and 4.388 pg/mL, respectively. Finally, in order to verify the feasibility of its clinical application, the serum samples of healthy subjects; cervical intraepithelial neoplasia I (CINI), CINII, and CINIII; and cervical cancer patients were analyzed, and the reliability of the results was confirmed by enzyme-linked immunosorbent assay experiments. The constructed SERS-based immunoassay platform could be used as a clinical tool for early screening of cancers in the future.









Similar content being viewed by others
References
Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–82.
Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17:64–84.
Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:72–83.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA-Cancer J Clin. 2019;69:7–34.
Jin YJ, Kim SC, Kim HJ, Ju W, Kim YH, Kim HJ. Use of autoantibodies against tumor-associated antigens as serum biomarkers for primary screening of cervical cancer. Oncotarget. 2017;62:105425–39.
Kaminska A, Sprynskyy M, Winkler K, Szymborski T. Ultrasensitive SERS immunoassay based on diatom biosilica for detection of interleukins in blood plasma. Anal Bioanal Chem. 2017;409:6337–47.
Zhao QY, Duan RQ, Yuan JL, Quan Y, Yang H, Xi MR. A reusable localized surface plasmon resonance biosensor for quantitative detection of serum squamous cell carcinoma antigen in cervical cancer patients based on silver nanoparticles array. Int J Nanomedicine. 2014;9:1097–104.
Zhou L, Liu Y, Wang FY, Jia ZJ, Zhou J, Jiang T, et al. Classification analyses for prostate cancer, benign prostate hyperplasia and healthy subjects by SERS-based immunoassay of multiple tumour markers. Talanta. 2018;188:238–44.
Zhou Y, Wang W, Wei R, Jiang GY, Li F, Chen X, et al. Serum bradykinin levels as a diagnostic marker in cervical cancer with a potential mechanism to promote VEGF expression via BDKRB2. Int J Oncol. 2019;55:131–41.
Chon H, Lee S, Yoon SY, Chang SI, Lima DW, Choo J. Simultaneous immunoassay for the detection of two lung cancer markers using functionalized SERS nanotags. Chem Commun. 2011;47:12515–7.
Cho HB, Hong SW, Oh YJ, Kim MA, Kang ES, Lee JM, et al. Clinical significance of osteopontin expression in cervical cancer. J Cancer Res Clin Oncol. 2008;134:909–17.
Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17–24.
Xu Q, Yuan BB, Xue FX, Zhang LQ, Li J, Guo HL, et al. OPN gene polymorphisms are associated with susceptibility and clinicopatholigical characteristics of cervical cancer in a Chinese cohort. Cancer Biomark. 2011;10:233–9.
Sharma P, Kumar S, Kundu GC. Transcriptional regulation of human osteopontin promoter by histone deacetylase inhibitor, trichostatin A in cervical cancer cells. Mol Cancer. 2010;9:178–90.
Mua GY, Wang HJ, Cai ZG, Ji H. OPN -443C>T genetic polymorphism and tumor OPN expression are associated with the risk and clinical features of papillary thyroid cancer in a Chinese cohort. Cell Physiol Biochem. 2013;32:171–9.
Cheng ZY, Choi N, Wang R, Lee S, Moon KC, Yoon SY, et al. Simultaneous detection of dual prostate specific antigens using surface-enhanced Raman scattering-based immunoassay for accurate diagnosis of prostate cancer. ACS Nano. 2017;11:4926–33.
Wang ZY, Zong SF, Wu L, Zhu D, Cui YP. SERS-activated platforms for immunoassay: probes, encoding methods, and applications. Chem Rev. 2017;117:7910–63.
Wang XK, Choi N, Cheng ZY, Ko JH, Chen LX, Choo J. Simultaneous detection of dual nucleic acids using a SERS-based lateral flow assay biosensor. Anal Chem. 2017;89:1163–9.
Li XZ, Yang TY, Song YT, Zhu JH, Wang DL, Li W. Surface-enhanced Raman spectroscopy (SERS)-based immunochromatographic assay (ICA) for the simultaneous detection of two pyrethroid pesticides. Sens Actuator B-Chem. 2019;283:230–8.
Zheng ZH, Wu L, Li L, Zong SF, Wang ZY, Cui YP. Simultaneous and highly sensitive detection of multiple breast cancer biomarkers in real samples using a SERS microfluidic chip. Talanta. 2018;188:507–15.
Tian Y, Liu H, Chen Y, Zhou C, Jiang Y, Gu C, et al. Seedless one-spot synthesis of 3D and 2D Ag nanoflowers for multiple phase SERS-based molecule detection. Sens Actuator B-Chem. 2019;301:127–42.
Zhao P, Li HX, Li DW, Hou YJ, Mao L, Yang M, et al. A SERS nano-tag-based magnetic-separation strategy for highly sensitive immunoassay in unprocessed whole blood. Talanta. 2019;198:527–33.
Wang Z, Yang HQ, Wang MH, Petti LC, Jiang T, Jia ZH, et al. SERS-based multiplex immunoassay of tumor markers using double SiO2@Ag immune probes and gold-film hemisphere array immune substrate. Colloid Surf A-Physicochem Eng Asp. 2018;546:48–58.
Fales AM, Yuan H, Vo-Dinh T. Development of hybrid silver-coated gold nanostars for nonaggregated surface-enhanced Raman scattering. J Phys Chem C. 2014;118:3708–15.
Lin S, Lin X, Liu YL, Zhao HY, Hasi W, Wang L. Self-assembly of Au@Ag core-shell nanocubes embedded with internal standard for reliable quantitative SERS measurements. Anal Methods. 2018;10:4201–8.
Khlebtsov BN, Khanadeev VA, Tsvetkov MY, Bagratashvili VN, Khlebtsov NG. Surface-enhanced Raman scattering substrates based on self-assembled PEGylated gold and gold-silver core-shell nanorods. J Phys Chem C. 2013;117:23162–71.
Tian YF, Zhou W, Yin BC, Ye BC. Highly sensitive surface-enhanced Raman scattering detection of adenosine triphosphate based on core-satellite assemblies. Anal Methods. 2017;9:6038–43.
Tan LQ, Liu C, Wang Y, Sun J, Dong J, Qian WP. Fabrication of SERS substrates containing dense “hot spots” by assembling star-shaped nanoparticles on superhydrophobic surfaces. New J Chem. 2017;41:5028–33.
Muhammad M, Shao CS, Huang Q. Label-free SERS diagnostics of radiation-induced injury via detecting the biomarker Raman signal in the serum and urine bio-samples based on Au-NPs array substrates. Spectroc Acta Pt A-Mol Biomol Spectr. 2019;223:117282–92.
Yoo J, Lee SW. Facile synthesis of silica-encapsulated gold nanoflowers as surface-enhanced Raman scattering probes using silane-mediated sol-gel reaction. J Nanosci Nanotechnol. 2016;16:6289–93.
Zhou L, Han ST, Shu SW, Zhuang JQ, Yan Y, Sun QJ, et al. Localized surface plasmon resonance-mediated charge trapping/detrapping for core-shell nanorod-based optical memory cells. ACS Appl Mater Interfaces. 2017;9:34101–10.
Bai TT, Sun JF, Che RC, Xu LN, Yin CY, Guo ZR, et al. Controllable preparation of core-shell Au-Ag nanoshuttles with improved refractive index sensitivity and SERS activity. ACS Appl Mater Interfaces. 2014;6:3331–40.
Yi SJ, Sun LM, Lenaghan SC, Wang YZ, Chong XY, Zhang ZL, et al. One-step synthesis of dendritic gold nanoflowers with high surface-enhanced Raman scattering (SERS) properties. RSC Adv. 2013;3:10139–44.
Sun G, Ye G, Wang K, Lou M, Jia X, Xu F, et al. Deposition of Ag films on liquid substrates via thermal evaporation for surface-enhanced Raman scattering. ACS Omega. 2020;5:7440–5.
Bu Y, Lee SW. Flower-like gold nanostructures electrodeposited on indium tin oxide (ITO) glass as a SERS-active substrate for sensing dopamine. Microchim Acta. 2015;182:1313–21.
Reokrungruang P, Chatnuntawech I, Dharakul T, Bamrungsap S. A simple paper-based surface enhanced Raman scattering (SERS) platform and magnetic separation for cancer screening. Sens Actuator B-Chem. 2019;285:462–9.
Xie J, Li LY, Khan IM, Wang ZP, Ma XY. Flexible paper-based SERS substrate strategy for rapid detection of methyl parathion on the surface of fruit. Spectroc Acta Pt A-Mol Biomol Spectr. 2020;2231:118104–12.
Linh VTN, Moon J, Mun CW, Devaraj V, Oh JW, Park SG, et al. A facile low-cost paper-based SERS substrate for label-free molecular detection. Sens Actuator B-Chem. 2019;291:369–77.
Zhang CM, You TT, Yang N, Gao YK, Jiang L, Yin PG. Hydrophobic paper-based SERS platform for direct-droplet quantitative determination of melamine. Food Chem. 2019;287:363–8.
Lee M, Oh K, Choi HK, Lee SG, Youn HJ, Lee HL, et al. Subnanomolar sensitivity of filter paper-based SERS sensor for pesticide detection by hydrophobicity change of paper surface. Sensors. 2018;3:151–9.
Han CQ, Li YQ, Jia Q, Bradley LH, Gan Y, Yao Y, et al. On-demand fabrication of surface-enhanced Raman scattering arrays by pen writing, and their application to the determination of melamine in milk. Microchim Acta. 2017;184:2909–17.
Lee DJ, Kim DY, Hydrophobic Paper-Based SERS. Sensor using gold nanoparticles arranged on graphene oxide flakes. Sensors. 2019;19:5471–80.
Zhao H, Hasi W, Li N, Sha XY, Lina S, Han S. In situ analysis of pesticide residues on the surface of agricultural products via surface-enhanced Raman spectroscopy using a flexible Au@Ag-PDMS substrate. New J Chem. 2019;43:13075–82.
Li M, Zhang ZS, Zhang X, Li KY, Yu XF. Optical properties of Au/Ag core/shell nanoshuttles. Opt Express. 2008;18:14288–93.
Liu K, Bai YC, Zhang L, Yang ZB, Fan QK, Zheng HQ, et al. Porous Au−Ag nanospheres with high-density and highly accessible hotspots for SERS analysis. Nano Lett. 2016;16:3675–81.
Liu F, Lu YH, Yu WH, Fu Q, Wang P, Ming H. Tunable surface-enhanced Raman spectroscopy via plasmonic coupling between nanodot-arrayed Ag film and Ag nanocube. Plasmonics. 2013;8:1279–84.
Baghbanian SM, Rezaei N, Tashakkorian H. Cheminform abstract: nanozeolite clinoptilolite as a highly efficient heterogeneous catalyst for the synthesis of various 2-amino-4H-chromene derivatives in aqueous media. Green Chem. 2014;15:3446–58.
Qi W, Zhou DB, Chen SL, Huang Y, Cheng X. Preparation and electrocatalytic properties of Fe, Co, Ni-polymer-C complex catalysts for ethanol electro-oxidation. Acta Chim Sin. 2009;67:917–22.
Nagase Y, Yoshida S, Kamiyama K. Analysis of human tear fluid by Fourier transform infrared spectroscopy. Biopolymers. 2005;79:18–27.
Liu KZ, Shaw RA, Man A, Dembinski TC, Mantsch HH. Reagent-free, simultaneous determination of serum cholesterol in HDL and LDL by infrared spectroscopy. Clin Chem. 2002;48:499–506.
Song CY, Yang YJ, Yang BY, Min LH, Wang LH. Combination assay of lung cancer associated serum markers using surface-enhanced Raman spectroscopy. J Mater Chem B. 2016;4:1811–7.
Funding
Financial support was from the National Natural Science Foundation of China (No. 81701825), the Social Development Foundation of Jiangsu (No. BE2018684), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No.17KJB416012), Yangzhou Science and Technology Project (No.YZ2017075), and Contract for Maternal and Child Health Research Projects in Jiangsu Province (No. F201809).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study was approved by the Ethics Committee of the College of Clinical Medicine of Yangzhou University and followed the guidelines of the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 730 kb)
Rights and permissions
About this article
Cite this article
Lu, D., Ran, M., Liu, Y. et al. SERS spectroscopy using Au-Ag nanoshuttles and hydrophobic paper-based Au nanoflower substrate for simultaneous detection of dual cervical cancer–associated serum biomarkers. Anal Bioanal Chem 412, 7099–7112 (2020). https://doi.org/10.1007/s00216-020-02843-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02843-x