Abstract
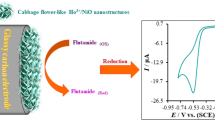
Cabbage flower–like Ho3+/NiO nanostructure (CFL-Ho3+/NiO NSs) with significant electrocatalytic oxidation has been published for the first time. First, structure and morphology of CFL-Ho3+/NiO-NSs have been described by XRD, SEM, and EDX methods. Then, CFL-Ho3+/NiO-NSs have been applied as a modifier for simultaneous electrochemical detection of methotrexate (MTX) and carbamazepine (CBZ). Functions of the modified electrode have been dealt with through electrochemical impedance spectroscopy (EIS). It has been demonstrated that the electrode response has been linear from 0.001–310.0 μM with a limit of detection of 5.2 nM and 4.5 nM (3 s/m) through DPV for MTX and CBZ. Diffusion coefficient (D) and heterogeneous rate constant (kh) have been detected for MTX and CBZ oxidation at the surface of the modified electrode. Moreover, CFL-Ho3+/NiO-NS/GCE has been employed for determining MTX and CBZ in urine and drug specimens. Outputs showed the analyte acceptable recovery. Therefore, the electrode could be applied to analyze both analytes in drug prescription and clinical laboratories.

Electrochemical sensor based on bifunctional cabbage flower–like Ho3+/NiO nanostructures modified glassy carbon electrode for simultaneous detecting methotrexate and carbamazepine was fabricated












Similar content being viewed by others
References
Asadian E, Shahrokhian S, Zad AI, Ghorbani-Bidkorbeh F. Glassy carbon electrode modified with 3D graphene–carbon nanotube network for sensitive electrochemical determination of methotrexate. Sensors Actuators B Chem. 2017;239:617–27.
Huang D, Wu H, Zhu Y, Su H, Zhang H, Sheng L, et al. Sensitive determination of anticancer drug methotrexate using graphite oxide-nafion modified glassy carbon electrode. Int J Electrochem Sci. 2019;14:3792–804.
Šelešovská R, Janíková-Bandžuchová L, Chýlková J. Sensitive voltammetric sensor based on boron-doped diamond electrode for determination of the chemotherapeutic drug methotrexate in pharmaceutical and biological samples. Electroanalysis. 2015;27:42–51.
Chen J, Fu B, Liu T, Yan Z, Li KA. graphene oxide-DNA electrochemical sensor based on glassy carbon electrode for sensitive determination of methotrexate. Electroanalysis. 2018;30:288–95.
Jagirani MS, Mahesar SA, Sherazi STH, Qureshi MS, Soomro RA, Lakho SA, et al. Electrochemical oxidation of methotrexate using pheniramine maleate functionalized gold nanoparticles modified electrode. Sens Lett. 2018;16:8–12.
Kummari S, Kumar VS, Satyanarayana M, Gobi KV. Direct electrochemical determination of methotrexate using functionalized carbon nanotube paste electrode as biosensor for in-vitro analysis of urine and dilute serum samples. Microchem J. 2019;148:626–33.
König A, Weidauer C, Seiwert B, Reemtsma T, Unger T, Jekel M. Reductive transformation of carbamazepine by abiotic and biotic processes. Water Res. 2016;101:272–80.
Balasubramanian P, Balamurugan TST, Chen SM, Chen TW, Ali MA, Al-Hemaid FM, et al. An amperometric sensor for low level detection of antidepressant drug carbamazepine based on graphene oxide-g-C3N4 composite film modified electrode. J Electrochem Soc. 2018;165:B160–6.
Teradale AB, Lamani SD, Ganesh PS, Swamy BEK, Das SN. Electrochemical sensor for the determination of paracetamol at carbamazepine film coated carbon paste electrode. Z Phys Chem. 2018;232:345–58.
Tarahomi S, Rounaghi GH, Zavar MHA, Daneshvar L. Electrochemical sensor based on tio2 nanoparticles/nafion biocompatible film modified glassy carbon electrode for carbamazepine determination in pharmaceutical and urine samples. J Electrochem Soc. 2018;165:B946–52.
Lavanya N, Sekar C, Ficarra S, Tellone E, Bonavita A, Leonardi SG, et al. A novel disposable electrochemical sensor for determination of carbamazepine based on Fe doped SnO2 nanoparticles modified screen-printed carbon electrode. Mater Sci Eng. 2016;62:53–60.
Ghasemian S, Nasuhoglu D, Omanovic S, Yargeau V. Photoelectrocatalytic degradation of pharmaceutical carbamazepine using Sb-doped Sn80%-W20%-oxide electrodes. Sep Purif Technol. 2017;188:52–9.
Hassaninejad-Darzi SK, Shajie F. Simultaneous determination of acetaminophen, pramipexole and carbamazepine by ZSM-5 nanozeolite and TiO2 nanoparticles modified carbon paste electrode. Mater Sci Eng. 2018;91:64–77.
Yang B, Deng J, Yu G, Deng S, Li J, Zhu C, et al. Effective degradation of carbamazepine using a novel electro-peroxone process involving simultaneous electrochemical generation of ozone and hydrogen peroxide. Electrochem Commun. 2018;86:26–9.
Gurung K, Ncibi MC, Shestakova M, Sillanpää M. Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode. Appl Catal B-Environ. 2018;221:329–38.
Kelmann R, Kuminek G, Teixeira H, Koester L. Determination of carbamazepine in parenteral nanoemulsions: development and validation of an HPLC method. Chromatographia. 2007;66:427–30.
Halwachs S, Lakoma C, Schafer I, Seibel P, Honscha W. The antiepileptic drugs phenobarbital and carbamazepine reduce transport of methotrexate in rat choroid plexus by down-regulation of the reduced folate carrier. Mol Pharmacol. 2011;80:621–9.
Behbahani M, Najafi F, Bagheri S, Bojdi MK, Salarian M, Bagheri A. Application of surfactant assisted dispersive liquid-liquid microextraction as an efficient sample treatment technique for preconcentration and trace detection of zonisamide and carbamazepine in urine and plasma samples. J Chromatogr A. 2013;1308:25–31.
Durán-Alvarez JC, Becerril-Bravo E, Castro VS, Jiménez B, Gibson R. The analysis of a group of acidic pharmaceuticals, carbamazepine, and potential endocrine disrupting compounds in wastewater irrigated soils by gas chromatography-mass spectrometry. Talanta. 2009;78:1159–66.
Marziali E, Raggi MA, Komarova N, Kenndler E. Octakis-6-sulfato-γ-cyclodextrin as additive for capillary electrokinetic chromatography of dibenzoazepines: carbamazepine, oxcarbamazepine and their metabolites. Electrophoresis. 2002;23:3020–6.
Maggs J, Pirmohamed M, Kitteringham N, Park B. Characterization of the metabolites of carbamazepine in patient urine by liquid chromatography/mass spectrometry. Drug Metab Dispos. 1997;25:275–80.
Lee SH, Li M, Suh JK. Determination of carbamazepine by chemiluminescence detection using chemically prepared tris (2, 2′-bipyridine)-ruthenium (III) as oxidant. Anal Sci. 2003;19:903–6.
Rezaei Z, Hemmateenejad B, Khabnadideh S, Gorgin M. Simultaneous spectrophotometric determination of carbamazepine and phenytoin in serum by PLS regression and comparison with HPLC. Talanta. 2005;65:21–8.
Gupta VK, Agarwal S, Singhal B. Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb Chem High Throughput Screen. 2011;14:284–302.
Gupta VK, Sethi B, Sharma RA, Agarwal S, Bharti A. Mercury selective potentiometric sensor based on low rim functionalized thiacalix [4] arene as a cationic receptor. J Mol Liq. 2013;177:114–8.
Rajaei M, Foroughi MM, Jahani S, Shahidi Zandi M, Hassani Nadiki H. Sensitive detection of morphine in the presence of dopamine with La3+ doped fern-like CuO nanoleaves/MWCNTs modified carbon paste electrode. J Mol Liq. 2019;284:462–72.
Gupta VK, Ganjali MR, Norouzi P, Khani H, Nayak A, Agarwal S. Electrochemical analysis of some toxic metals and drugs by ion selective electrodes. Crit Rev Anal Chem. 2011;41:282–313.
Srivastava SK, Gupta VK, Jain S. Determination of lead using poly (vinyl chloride) based crown ether membrane. Analyst. 1995;120:495–8.
Chekin F, Myshin V, R, Melinte S, Singh SK, Kurungot S, Boukherroub R, Szunerits S, Graphene-modified electrodes for sensing doxorubicin hydrochloride in human plasma. Anal Bioanal Chem 2019; 411: 1509–1516.
Jain AK, Gupta VK, Sahoo BB, Singh LP. Copper(II)-selective electrodes based on macrocyclic compounds. Anal Proc Incl Anal Commun. 1995;32:99–101.
Gupta VK, Karimi-Maleh H, Sadegh R. Simultaneous determination of hydroxylamine, phenol and sulfite in water and waste water samples using a voltammetric nanosensor. J Electrochem Sci. 2015;10:303–16.
Foroughi MM, Jahani S, Hasani Nadiki H. Lanthanium doped fern-like CuO nanoleaves/MWCNTs modified glassy carbon electrode for simultaneous determination of tramadol and acetaminophen. Sensors Actuators B Chem. 2019;285:562–70.
Gupta VK, Singh AK, Kumawat LK. Thiazole Schiff base turn-in fluorescent chemosensor for Al3+ ion. Sensors Actuators B Chem. 2014;195:98–108.
Srivastava SK, Gupta VK, Jain S. PVC-based 2,2,2-cryptand sensors for zinc ions. Anal Chem. 1996;68:1272–5.
Rasa Pauliukaite R, Metelka R, Švancara I, Królicka A, Bobrowski A, Vytřas K, et al. Carbon paste electrodes modified with Bi2O3 as sensors for the determination of Cd and Pb. Anal Bioanal Chem. 2002;374:1155–8.
Gupta VK, Pathania D, Agarwal S, Sharma S. Decolorization of hazardous dye from water system using chemical modified Ficus carica adsorbent. J Mol Liq. 2012;174:86–94.
Gupta VK, Kumar S, Singh R, Singh LP, Shoora SK, Sethi B. Cadmium (II) ion sensing through p-tert-butyl calix[6]arene based potentiometric sensor. J Mol Liq. 2014;195:65–8.
Safaei M, Foroughi MM, Ebrahimpoor N, Jahani S, Omidi A, Khatami M. A review on metal-organic frameworks: synthesis and applications. Trends Anal Chem. 2019;118:401–26.
Karthikeyan S, Boopathy R, Titus A, Sekaran G. A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies. J Mol Liq. 2012;173:153–63.
Dehghani MH, Sanaei D, Ali I, Bhatnagar A. Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: kinetic modeling and isotherm studies. J Mol Liq. 2016;215:671–9.
Qian P, Qin Y, Lyu Y, Li Y, Wang L, Wang S, et al. A hierarchical cobalt/carbon nanotube hybrid nanocomplex-based ratiometric fluorescent nanosensor for ultrasensitive detection of hydrogen peroxide and glucose in human serum. Anal Bioanal Chem. 2019;411:1517–24.
Asfaram A, Ghaedi M, Agarwal S, Tyagi L, Gupta VK. Removal of basic dye Auramine-O by ZnS:Cu nanoparticles loaded on activated carbon: optimization of parameters using response surface methodology with central composite design. RSC Adv. 2015;5:18438–50.
Gupta VK, Atar N, Yola ML, Üstündağ Z, Uzun L. A novel magnetic Fe@Au core–shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res. 2014;48:210–7.
Torkzadeh-Mahani R, Foroughi MM, Jahani S, Kazemipour M, Hassani Nadiki H. The effect of ultrasonic irradiation on the morphology of NiO/Co3O4 nanocomposite and its application to the simultaneous electrochemical determination of droxidopa and carbidopa. Ultrason Sonochem. 2019;56:183–92.
Yola ML, Gupta VK, Eren T, Emre Şen A, Atar N. A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim Acta. 2014;120:204–11.
Gupta VK, Mergu VN, Kumawat LK, Singh AK. Selective naked-eye detection of magnesium(II) ions using a coumarin-derived fluorescent probe. Sensors Actuators B Chem. 2015;207:216–23.
Jahani S. Evaluation of the usefulness of an electrochemical sensor in detecting ascorbic acid using a graphite screen-printed electrode modified with NiFe2O4 nanoparticles. Anal Bioanal Electrochem. 2018;10:739–50.
Gupta VK, Mergu N, Kumawat LK, Singh AK. A reversible fluorescence “off-on-off” sensor for sequential detection of aluminum and acetate/fluoride ions. Talanta. 2015;144:80–9.
Karimi-Maleh H, Tahernejad-Javazmi F, Atar N, Yola ML, Gupta VK, Ensafi AA. A novel DNA biosensor based on a pencil graphite electrode modified with polypyrrole/functionalized multiwalled carbon nanotubes for determination of 6-mercaptopurine anticancer drug. Ind Eng Chem Res. 2015;54:3634–9.
Mirzaei H, Nasiri AK, Mohamadee R, Yaghoobi H, Khatami M, Azizi O, et al. Direct growth of ternary copper nickel cobalt oxide nanowires as binder-free electrode on carbon cloth for nonenzymatic glucose sensing. Microchem J. 2018;142:343–51.
Jain AK, Gupta VK, Singh LP. Neutral carrier and organic resin based membranes as sensors for uranyl ions. Anal Proc Incl Anal Commun. 1995;32:263–5.
Ghanei-Motlagh M, Taher MA, Heydari A, Ghanei-Motlagh R, Gupta VK. A novel voltammetric sensor for sensitive detection of mercury (II) ions using glassy carbon electrode modified with graphene-based ion imprinted polymer. Mater Sci Eng C. 2016;63:367–75.
Arefi Nia N, Foroughi MM, Jahani S, Shahidi Zandi M, Rastakhiz N. Fabrication of a new electrochemical sensor for simultaneous determination of codeine and diclofenac using synergic effect of feather-type La3+-ZnO nano-flower. J Electrochem Soc. 2019;166:B489–97.
Goval RN, Gupta VK, Sangal A, Bachheti N. Voltammetric determination of uric acid at a fullerene-C60-modified glassy carbon electrode. Electroanalysis. 2005;17:2217–23.
Gupta VK, Kumar P. Cadmium (II)-selective sensors based on dibenzo-24-crown-8 in PVC matrix. Anal Chim Acta. 1999;389:205–12.
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM. Nanosized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature. 2000;407:496–9.
Yin XT, Zhou WD, Li J, Wang Q, Wu FY, Dastan D, et al. A highly sensitivity and selectivity Pt-SnO2 nanoparticles for sensing applications at extremely low level hydrogen gas detection. J Alloys Compd. 2019;805:229–36.
Khatami M, Heli H, Mohammadzadeh Jahani P, Azizi H, Nobre MAL. Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. IET Nanobiotechnol. 2017;11:709–13.
Yin XT, Zhou WD, Li J, Lv P, Wang Q, Wang D, et al. Tin dioxide nanoparticles with high sensitivity and selectivity for gas sensors at sub-ppm level of hydrogen gas detection. J Mater Sci Mater Electron. 2019;30:14687–94.
Khatami M, Nejad MS, Salari S, Almani PGN. Plant-mediated green synthesis of silver nanoparticles using Trifolium resupinatum seed exudate and their antifungal efficacy on Neofusicoccum parvum and Rhizoctonia solani. IET Nanobiotechnol. 2016;10:237–43.
Wang J, Zeng W, Wang Z. Assembly of 2D nanosheets into 3D flower-like NiO: synthesis and the influence of petal thickness on gas-sensing properties. Ceram Int. 2016;42:4567–73.
Needham SA, Wang GX, Liu HK. Synthesis of NiO nanotubes for use as negative electrodes in lithium ion batteries. J Power Sources. 2006;159:254–7.
Zhu LP, Liao GH, Yang Y, Xiao HM, Wang JF, Fu SY. Selfassembled 3D flower-like hierarchical b-Ni(OH)2 hollow architectures and their in situ thermal conversion to NiO. Nanoscale Res Lett. 2009;4:550–7.
Li Q, Chen Y, Yang T, Lei D, Zhang G, Mei L, et al. Preparation of 3D flower-like NiO hierarchical architectures and their electrochemical properties in lithium-ion batteries. Electrochim Acta. 2013;90:80–9.
Zhang F, Wang X, Zhang X, Turxun M, Yu H, Zhao J. The catalytic activity of NiO for N2O decomposition doubly promoted by barium and cerium. Chem Eng J. 2014;256:365–71.
Bloemen M, Vandendriessche S, Goovaerts V, Brullot W, Vanbel M, Carron S, et al. Synthesis and characterization of holmium-doped iron oxide nanoparticles. Materials. 2014;7:1155–64.
Bard A, Faulkner L. Electrochemical methods fundamentals and applications. 2nd ed. New York: Wiley; 2001.
Foroughi MM, Jahani S, Rajaei M. Facile fabrication of 3D dandelion-like cobalt oxide nanoflowers and its functionalization in the first electrochemical sensing of oxymorphone: evaluation of kinetic parameters at the surface electrode. J Electrochem Soc. 2019;166:B1300–11.
Iranmanesh T, Foroughi MM, Jahani S, Shahidi Zandi M, Hassani Nadiki H. Green and facile microwave solvent-free synthesis of CeO2 nanoparticle-decorated CNTs as a quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen. Talanta. 2020;207:120318.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study has been approved by the Islamic Azad University of Kerman Branch ethics committee and has been performed in accordance with the ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fathi, Z., Jahani, S., Zandi, M.S. et al. Synthesis of bifunctional cabbage flower–like Ho3+/NiO nanostructures as a modifier for simultaneous determination of methotrexate and carbamazepine. Anal Bioanal Chem 412, 1011–1024 (2020). https://doi.org/10.1007/s00216-019-02326-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02326-8