Abstract
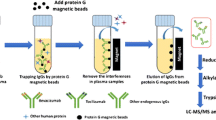
To comprehensively evaluate the pharmacokinetic (PK) characteristics of aflibercept, we established a liquid chromatography with tandem mass spectrometry (LC-MS/MS) method to determine the concentration of vascular endothelial growth factor (VEGF)-A-bound aflibercept and free aflibercept. A specific sample preparation method of nano-surface and molecular-orientation limited (nSMOL) proteolysis was performed to extract both free and bound aflibercept from plasma. The tryptic peptides unique to aflibercept and VEGF-A were selected to quantify the amounts of total aflibercept and aflibercept–VEGF complex, respectively. The method was validated by evaluating its selectivity, linearity, precision, accuracy, extraction recovery, matrix effect, and stability. It was then successfully used to quantify total and bound aflibercept concentrations in cynomolgus monkey plasma, while indirectly obtaining the concentration of free aflibercept by subtraction. The PK results of this LC-MS/MS method are comparable to the traditional enzyme-linked immunosorbent assay (ELISA) results. It is thus a reliable and complementary method for the PK evaluation of aflibercept.

Graphical abstract



Similar content being viewed by others
References
Vempati P, Popel AS, Gabhann FM. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25(1):1–19.
Napoleone F, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–74.
Do DV, Quan DN, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, et al. One-year outcomes of the DA VINCI study of VEGF trap-eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658–65.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. Ophthalmology. 2016;123(6):1351–9.
Ho AC, Scott IU, Kim SJ, Brown GC, Brown MM, Ip MS, et al. Anti–vascular endothelial growth factor pharmacotherapy for diabetic macular edema : a report by the American Academy of Ophthalmology. Ophthalmology. 2012;119(10):2179–88.
Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE, et al. Five-year outcomes with anti–vascular endothelial growth factor treatment of neovascular age-related macular degeneration : the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751–61.
Rudge JS, Holash J, Hylton D, Russell M, Jiang S, Leidich R, et al. VEGF trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A. 2007;104(47):18363–70.
Thai HT, Veyrat-Follet C, Vivier N, Dubruc C, Sanderink G, Mentré F, et al. A mechanism-based model for the population pharmacokinetics of free and bound aflibercept in healthy subjects. Br J Clin Pharmacol. 2011;72(3):402–14.
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–8.
Holash J, Rudge J, Davis S, Papadopoulos N, Wiegand S, Yancopoulos G, et al. VEGF-trap: a novel, potent VEGF blocker with anti-tumor effects. Eur J Cancer. 2003;38(17):S82.
Sarwar S, Bakbak B, Sadiq MA, Sepah YJ, Shah SM, Ibrahim M, et al. Fusion proteins: aflibercept (VEGF trap-eye). Dev Ophthalmol. 2016;55:282.
Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF trap. Br J Ophthalmol. 2015;92(5):667–8.
Walker A, Chung CW, Neu M, Burman M, Batuwangala T, Jones G, et al. Novel interaction mechanism of a domain antibody-based inhibitor of human vascular endothelial growth factor with greater potency than ranibizumab and bevacizumab and improved capacity over aflibercept*. J Biol Chem. 2016;291(11):5500–11.
Celik N, Scheulerle A, Auffarth GU, Kopitz J, Dithmar S. Intraocular pharmacokinetics of aflibercept and vascular endothelial growth factor-A. Invest Ophth Vis Sci. 2015;56(9):5574.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal Aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48.
Folprecht G, Pericay C, Saunders MP, Thomas A, Zalcberg JR. Oxaliplatin and 5-FU/folinic acid (modified FOLFOX6) with or without aflibercept in first-line treatment of patients with metastatic colorectal cancer: the AFFIRM study. Ann Oncol. 2016;9(11):1273–9.
Zhang Q, Spellman DS, Song Y, Choi B, Hatcher NG, Tomazela D, et al. Generic automated method for liquid chromatography-multiple reaction monitoring mass spectrometry based monoclonal antibody quantitation for preclinical pharmacokinetic studies. Anal Chem. 2014;86(17):8776–84.
Heinig K, Wirz T, Schick E, Guenzi A. Bioanalysis of therapeutic peptides: differentiating between total and anti-drug antibody bound drug using liquid chromatography–tandem mass spectrometry quantitation. J Chromatogr A. 2013;1316(21):69–77.
Zheng O, Furlong MT, Steven W, Bogdan S, James T, Haiqing W, et al. Pellet digestion: a simple and efficient sample preparation technique for LC-MS/MS quantification of large therapeutic proteins in plasma. Bioanalysis. 2012;4(1):17–28.
Jiang H, Cao H, Zhang Y, Fast DM. Systematic evaluation of supported liquid extraction in reducing matrix effect and improving extraction efficiency in LC-MS/MS based bioanalysis for 10 model pharmaceutical compounds. J Chromatogr B. 2012;891–892(891–892):71–80.
Vialaret J, Broutin S, Pugnier C, Santelé S, Jaffuel A, Barnes A, et al. What sample preparation should be chosen for targeted MS monoclonal antibody quantification in human serum? Bioanalysis. 2018;10(7):723–35.
Iwamoto N, Shimomura A, Tamura K, Hamada A, Shimada T. LC-MS bioanalysis of Trastuzumab and released emtansine using nano-surface and molecular-orientation limited (nSMOL) proteolysis and liquid–liquid partition in plasma of Trastuzumab emtansine-treated breast cancer patients. J Pharm Biomed Anal. 2017;145:33–9.
Iwamoto N, Umino Y, Aoki C, Yamane N, Hamada A, Shimada T. Fully validated LCMS bioanalysis of Bevacizumab in human plasma using nano-surface and molecular-orientation limited (nSMOL) proteolysis. Drug Metab Pharmacokinet. 2016;31(1):46–50.
Iwamoto N, Yokoyama K, Takanashi M. Application of nSMOL coupled with LC-MS bioanalysis for monitoring the Fc-fusion biopharmaceuticals Etanercept and Abatacept in human serum. Pharmacol Res Perspect. 2018;6(4):e00422.
Iwamoto N, Yonezawa A, Matsubara K, Shimada T. Acceleration of nano-surface and molecular-orientation limited (nSMOL) proteolysis with acidified reduction pretreatment for quantification of Tocilizumab. J Pharm Biomed Anal. 2019;164:467–74.
Services USDoHaH. Bioanalytical method validation guidance for industry. Rockville: Food and Drug Administration; 2018.
Tew WP, Gordon M, Murren J, Dupont J, Pezzulli S, Aghajanian C, et al. Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors. Clin Cancer Res. 2010;16(1):358.
Kut C, MacGabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97(7):978.
Wang SJ, Wu ST, Gokemeijer J, Fura A, Krishna M, Morin P, et al. Attribution of the discrepancy between ELISA and LC-MS/MS assay results of a PEGylated scaffold protein in post-dose monkey plasma samples due to the presence of anti-drug antibodies. Anal Bioanal Chem. 2012;402(3):1229–39.
Acknowledgments
The authors would like to thank Shimadzu Co., Ltd., for their technical help.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No.81773679) and the project of Graduate Innovation Foundation of Yantai University, GIFYTU (No.YDZD1917).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study had been approved by the Institutional Animal Care and Use Committee with approval number IACUC-A2017033-K001-01.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 315 kb)
Rights and permissions
About this article
Cite this article
Kong, L., Liu, F., Huo, L. et al. A novel LC-MS/MS approach to the pharmacokinetic study of free and bound aflibercept simultaneously. Anal Bioanal Chem 412, 1003–1010 (2020). https://doi.org/10.1007/s00216-019-02316-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02316-w