Abstract
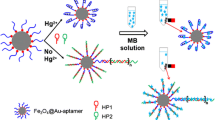
Given the gigantic harmfulness of bisphenol A (BPA), a novel and ultrasensitive aptasensor, which employs the truncated BPA aptamer, click chemistry, and activators generated by electron transfer for atom transfer radical polymerization (AGET ATRP), was developed herein for the quantitative determination of BPA. Firstly, hairpin DNAs (hairpins) with a thiol at the 5′ end and an azide group at the 3′ end were conjugated with aminated magnetic beads (MBs) through heterobifunctional cross-linkers. BPA truncated aptamer (ssDNA-A) hybridizes with its complementary single-stranded DNA (ssDNA-B) to form double-stranded DNA. In the presence of BPA, ssDNA-A specifically captures BPA, and then ssDNA-B is released. Subsequently, the ssDNA-B hybridizes with hairpins to expose the azide group near the surface of the MBs. Then, propargyl-2-bromoisobutyrate (PBIB), the initiator of AGET ATRP containing alkynyl group, was conjugated with azide group of hairpins via the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC). Consequently, a large number of fluorescein-o-acrylate (FA) were introduced to the MBs through AGET ATRP, resulting in that the fluorescence intensity was increased dramatically. Obviously, the fluorescence intensity was especially sensitive to the change of BPA concentration, and this method can be used in quantitative determination of BPA. Under optimal conditions, a broad liner range from 100 fM to 100 nM and a low limit of detection (LOD) of 6.6 fM were obtained. Moreover, the method exhibits not only excellent specificity for BPA detection over BPA analogues but high anti-interference ability in real water sample detection, indicating that it has huge application prospect in food safety and environment monitoring.







Similar content being viewed by others
References
Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–55.
Yildirim N, Long F, He M, et al. A portable optic fiber aptasensor for sensitive, specific and rapid detection of bisphenol-A in water samples. Environ Sci: Processes Impacts. 2014;16(6):1379–86.
Vogel SA. The politics of plastics: the making and unmaking of bisphenol a “safety”. Am J Public Health. 2009;99(S3):S559–66.
Flint S, Markle T, Thompson S, et al. Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manag. 2012;104:19–34.
Guida M, Troisi J, Ciccone C, et al. Bisphenol A and congenital developmental defects in humans. Mutat Res Fundam Mol Mech Mutagen. 2015;774:33–9.
Tharp AP, Maffini MV, Hunt PA, et al. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci. 2012;109(21):8190–5.
Lehmler HJ, Liu B, Gadogbe M, et al. Exposure to bisphenol A, bisphenol F, and bisphenol S in US adults and children: the national health and nutrition examination survey 2013–2014. ACS omega. 2018;3(6):6523–32.
Huysman S, Van Meulebroek L, Janssens O, et al. Targeted quantification and untargeted screening of alkylphenols, bisphenol A and phthalates in aquatic matrices using ultra-high-performance liquid chromatography coupled to hybrid Q-Orbitrap mass spectrometry. Anal Chim Acta. 2019;1049:141–51.
Rezaee M, Yamini Y, Shariati S, et al. Dispersive liquid–liquid microextraction combined with high-performance liquid chromatography-UV detection as a very simple, rapid and sensitive method for the determination of bisphenol A in water samples. J Chromatogr A. 2009;1216(9):1511–4.
Goeury K, Duy SV, Munoz G, et al. Analysis of Environmental Protection Agency priority endocrine disruptor hormones and bisphenol A in tap, surface and wastewater by online concentration liquid chromatography tandem mass spectrometry. J Chromatogr A. 2019;1591:87–98.
Liu A, Shen Z, Yuan L, et al. High-performance thin-layer chromatography coupled with HPLC-DAD/HPLC-MS/MS for simultaneous determination of bisphenol A and nine brominated analogs in biological samples. Anal Bioanal Chem. 2019;411(3):725–34.
Deceuninck Y, Bichon E, Marchand P, et al. Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015;407(9):2485–97.
Xiao Y, Lubin AA, Heeger AJ, et al. Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew Chem. 2010;117(34):5592–5.
Munzar JD, Ng A, Juncker D. Duplexed aptamers: history, design, theory, and application to biosensing. Chem Soc Rev. 2019;48(5):1390–419.
Kwon YS, Raston NHA, Gu MB. An ultra-sensitive colorimetric detection of tetracyclines using the shortest aptamer with highly enhanced affinity. Chem Commun. 2014;50(1):40–2.
Abnous K, Danesh NM, Ramezani M, et al. A novel electrochemical sensor for bisphenol A detection based on nontarget-induced extension of aptamer length and formation of a physical barrier. Biosens Bioelectron. 2018;119:204–8.
Jo M, Ahn JY, Lee J, et al. Development of single-stranded DNA aptamers for specific bisphenol A detection. Oligonucleotides. 2011;21(2):85–91.
Zhu Y, Cai Y, Xu L, et al. Building an aptamer/graphene oxide FRET biosensor for one-step detection of bisphenol A. ACS Appl Mater Interfaces. 2015;7(14):7492–6.
Lee ES, Kim GB, Ryu SH, et al. Fluorescing aptamer-gold nanosensors for enhanced sensitivity to bisphenol A. Sensors Actuators B Chem. 2018;260:371–9.
Deiminiat B, Rounaghi GH, Arbab-Zavar MH, et al. A novel electrochemical aptasensor based on f-MWCNTs/AuNPs nanocomposite for label-free detection of bisphenol A. Sensors Actuators B Chem. 2017;242:158–66.
Xue F, Wu J, Chu H, et al. Electrochemical aptasensor for the determination of bisphenol A in drinking water. Microchim Acta. 2013;180(1–2):109–15.
Marks HL, Pishko MV, Jackson GW, et al. Rational design of a bisphenol A aptamer selective surface-enhanced Raman scattering nanoprobe. Anal Chem. 2014;86(23):11614–9.
Gao P, Wang H, Li P, et al. In-site synthesis molecular imprinting Nb2O5–based photoelectrochemical sensor for bisphenol A detection. Biosens Bioelectron. 2018;121:104–10.
Mei Z, Chu H, Chen W, Xue F, Liu J, Xu H, et al. Ultrasensitive one-step rapid visual detection of bisphenol A in water samples by label-free aptasensor. Biosens Bioelectron. 2013;39(1):26–30.
Lee EH, Lim HJ, Lee SD, et al. Highly sensitive detection of bisphenol A by NanoAptamer assay with truncated aptamer. ACS Appl Mater Interfaces. 2017;9(17):14889–98.
Sun H, Qiu Y, Liu Q, Wang Q, et al. Ultrasensitive DNA biosensor based on electrochemical atom transfer radical polymerization. Biosens Bioelectron. 2019;131:193–9.
Hu Q, Kong J, Han D, Zhang Y, Bao Y, Zhang X, et al. Electrochemically controlled RAFT polymerization for highly sensitive electrochemical biosensing of protein kinase activity. Anal Chem. 2019;91(3):1936–43.
Yun W, Wu H, Chen L, Yang L. Dual enzyme-free amplification strategy for ultra-sensitive fluorescent detection of bisphenol A in water. Anal Chim Acta. 2018;1020:104–9.
Azizi M, Zaferani M, Cheong SH, et al. Pathogenic bacteria detection using RNA-based loop-mediated isothermal-amplification-assisted nucleic acid amplification via droplet microfluidics. ACS Sens. 2019;4(4):841–8.
Chen J, Zhou S. Label-free DNA Y junction for bisphenol A monitoring using exonuclease III-based signal protection strategy. Biosens Bioelectron. 2016;77:277–83.
Min K, Gao H, Matyjaszewski K. Preparation of homopolymers and block copolymers in miniemulsion by ATRP using activators generated by electron transfer (AGET). J Am Chem Soc. 2005;127(11):3825–30.
Werner A, Schmitt V, Sèbe G, et al. Convenient synthesis of hybrid polymer materials by AGET-ATRP polymerization of pickering emulsions stabilized by cellulose nanocrystals grafted with reactive moieties. Biomacromolecules. 2018;20(1):490–501.
Vidiella del Blanco M, Gomez V, Keplinger T, et al. Solvent-controlled spatial distribution of SI-AGET-ATRP grafted polymers in lignocellulosic materials. Biomacromolecules. 2018;20(1):336–46.
Liu X, Chen Q, Yang G, et al. Magnetic nanomaterials with near-infrared pH-activatable fluorescence via iron-catalyzed AGET ATRP for tumor acidic microenvironment imaging. J Mater Chem B. 2015;3(14):2786–800.
Wang C, Li Y, Wei Y. A sandwich boronate affinity sorbent assay for glucose detection facilitated by boronic acid-terminated fluorescent polymers. Sensors Actuators B Chem. 2017;247:595–601.
Lei YM, Zhou J, Chai YQ, et al. SnS2 quantum dots as new emitters with strong electrochemiluminescence for ultrasensitive antibody detection. Anal Chem. 2018;90(20):12270–7.
Zhao Z, Zhang M, Hogle JM, et al. DNA-corralled nanodiscs for the structural and functional characterization of membrane proteins and viral entry. J Am Chem Soc. 2018;140(34):10639–43.
Yu J, Lu C, Wang C, Wang J, Fan Y, Chu F. Sustainable thermoplastic elastomers derived from cellulose, fatty acid and furfural via ATRP and click chemistry. Carbohydr Polym. 2017;176:83–90.
Navarro LA, French DL, Zauscher S. Synthesis of modular brush polymer–protein hybrids using diazotransfer and copper click chemistry. Bioconjug Chem. 2018;29(8):2594–605.
Pan X, Fantin M, Yuan F, Matyjaszewski K. Externally controlled atom transfer radical polymerization. Chem Soc Rev. 2018;47(14):5457–90.
Wu Y, Liu S, He L. Electrochemical biosensing using amplification-by-polymerization. Anal Chem. 2009;81(16):7015–21.
Fantin M, Isse AA, Gennaro A, Matyjaszewski K. Understanding the fundamentals of aqueous ATRP and defining conditions for better control. Macromolecules. 2015;48(19):6862–75.
Xu J, Lee ES, Gye MC, Kim YP. Rapid and sensitive determination of bisphenol A using aptamer and split DNAzyme. Chemosphere. 2019;228:110–6.
Mo F, Xie J, Wu T, et al. A sensitive electrochemical sensor for bisphenol A on the basis of the AuPd incorporated carboxylic multi-walled carbon nanotubes. Food Chem. 2019;292:253–9.
Li Q, Bai J, Ren S, Wang J, Gao Y, Li S, et al. An ultrasensitive sensor based on quantitatively modified upconversion particles for trace bisphenol A detection. Anal Bioanal Chem. 2019;411(1):171–9.
Funding
This work was supported by the project of tackling of key scientific and technical problems in Henan Province (192102310033) and the National Natural Science Foundation of China (21575066).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, Z., Tang, J., Li, M. et al. An ultrasensitive fluorescent aptasensor based on truncated aptamer and AGET ATRP for the detection of bisphenol A. Anal Bioanal Chem 411, 7807–7815 (2019). https://doi.org/10.1007/s00216-019-02179-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02179-1