Abstract
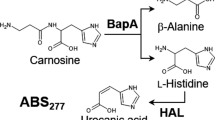
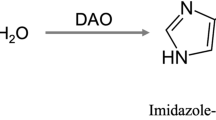
Intestinal diamine oxidase (DAO) acts as a protective barrier against exogenous histamine. A deficit of DAO activity can lead to the appearance of histamine intolerance, a clinical condition that may be treated by a low-histamine diet and oral DAO supplementation to enhance intestinal histamine degradation. As sources of DAO, porcine kidneys and certain legume seedlings are suitable components for the formulation of a DAO supplement. The aim of this work was to develop a rapid and reliable methodology for the in vitro determination of DAO activity in food matrices based on an enzymatic assay coupled to UHPLC-FL. The proposed method showed a satisfactory linearity and sensitivity and provided a relative standard deviation lower than 3%, guaranteeing method precision, and a mean recovery greater than 99% both for lyophilized pea sprouts and porcine kidney protein extracts. A high specificity is a key attribute of this method due to the use of histamine as the reaction substrate and the direct quantification of its degradation. Moreover, the lack of interference of catalase and hydrogen peroxide is another advantage in comparison with previously published methods. Lyophilized pea sprouts showed the greatest histamine-degrading activity (0.40 ± 0.01 mU/mg), followed by porcine kidney protein extracts (0.23 ± 0.01 mU/mg) and commercial DAO supplements (0.09 ± 0.06 mU/mg). This technique could be used as a tool to validate the DAO activity of food matrices of potential interest for the treatment of histamine intolerance.



Similar content being viewed by others
References
Best CH. The disappearance of histamine from autolysing lung tissue. J Physiol. 1929;67:256–63.
Mondovì B, Rotilio G, Finazzi A, Scioscia-Santoro A. Purification of pig-kidney diamine oxidase and its identity with histaminase. Biochem J. 1964. https://doi.org/10.1042/bj0910408.
Wolvekamp MC, de Bruin RW. Diamine oxidase: an overview of historical, biochemical and functional aspects. Dig Dis. 1994. https://doi.org/10.1159/000171432.
Elmore BO, Bollinger JA, Dooley DM. Human kidney diamine oxidase: heterologous expression, purification, and characterization. J Biol Inorg Chem. 2002. https://doi.org/10.1007/s00775-001-0331-1.
Masini E, Bani D, Marzocca C, Mateescu MA, Mannaioni PF, Federico R, et al. Pea seedling histaminase as a novel therapeutic approach to anaphylactic and inflammatory disorders. A plant histaminase in allergic asthma and ischemic shock. Sci World J. 2007; https://doi.org/10.1100/tsw.2007.139.
Finney J, Moon HJ, Ronnebaum T, Lantz M, Mure M. Human copper-dependent amine oxidases. Arch Biochem Biophys. 2014. https://doi.org/10.1016/j.abb.2013.12.022.
Kivirand K, Sõmerik H, Oldekop ML, Rebane R, Rinken T. Effect of spermidine and its metabolites on the activity of pea seedlings diamine oxidase and the problems of biosensing of biogenic amines with this enzyme. Enzym Microb Technol. 2016. https://doi.org/10.1016/j.enzmictec.2015.09.007.
Razali NN, Hashim NH, ATC L, Salleh AB. Conformational design and characterisation of a truncated diamine oxidase from Arthrobacter globiformis. High Throughput. 2018. https://doi.org/10.3390/ht7030021.
Schwelberger HG, Bodner E. Purification and characterization of diamine oxidase from porcine kidney and intestine. Biochim Biophys Acta. 1997;1340:152–64.
Naila A, Flint S, Fletcher GC, Bremer PJ, Meerdink G, Morton RH. Prediction of the amount and rate of histamine degradation by diamine oxidase (DAO). Food Chem. 2012. https://doi.org/10.1016/j.foodchem.2012.07.022.
Schwelberger HG, Feurle J, Houen G. New tools for studying old questions: antibodies for human diamine oxidase. J Neural Transm. 2013. https://doi.org/10.1007/s00702-012-0936-2.
Aschenbach JR, Schwelberger HG, Ahrens F, Fürll B, Gäbel G. Histamine inactivation in the colon of pigs in relationship to abundance of catabolic enzymes. Scand J Gastroenterol. 2006. https://doi.org/10.1080/00365520500419540.
Calinescu C, Federico R, Mondovi B, Mateescu MA. Zymographic assay of plant diamine oxidase on entrapped peroxidase polyacrylamide gel electrophoresis. A study of stability to proteolysis. Anal Bioanal Chem. 2010; https://doi.org/10.1007/s00216-009-3306-7.
Kovacova-Hanuskova E, Buday T, Gavliakova S, Plevkova J. Histamine, histamine intoxication and intolerance. Allergol Immunopathol. 2015. https://doi.org/10.1016/j.aller.2015.05.001.
Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007. https://doi.org/10.1093/ajcn/85.5.1185.
Comas-Basté O, Latorre-Moratalla ML, Bernacchia R, Veciana-Nogués MT, Vidal-Carou MC. New approach for the diagnosis of histamine intolerance based on the determination of histamine and methylhistamine in urine. J Pharm Biomed Anal. 2017. https://doi.org/10.1016/j.jpba.2017.06.029.
Jarisch R, Wantke F, Raithel M, Hemmer W. Histamine and biogenic amines. In: Jarisch R, editor. Histamine intolerance. Histamine and Seasickness. Heidelberg: Springer Verlag GmbH; 2015. p. 3–44.
Sánchez-Pérez S, Comas-Basté O, Rabell-González J, Veciana-Nogués MT, Latorre-Moratalla ML, Vidal-Carou MC. Biogenic amines in plant-origin foods: are they frequently underestimated in low-histamine diets? Foods. 2018. https://doi.org/10.3390/foods7120205.
EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9:2393.
Latorre-Moratalla ML, Comas-Baste O, Bover-Cid S, Vidal-Carou MC. Tyramine and histamine risk assessment related to consumption of dry fermented sausages by the Spanish population. Food Chem Toxicol. 2017. https://doi.org/10.1016/j.fct.2016.11.011.
Jumarie C, Séïde M, Marcocci L, Pietrangeli P, Mateescu MA. Diamine oxidase from white pea (Lathyrus sativus) combined with catalase protects the human intestinal Caco-2 cell line from histamine damage. Appl Biochem Biotechnol. 2017. https://doi.org/10.1007/s12010-016-2390-3.
Izquierdo-Casas J, Comas-Basté O, Latorre-Moratalla ML, Lorente-Gascón M, Duelo A, Soler-Singla L, et al. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin Nutr. 2019. https://doi.org/10.1016/j.clnu.2018.01.013.
Blemur L, Le TC, Marcocci L, Pietrangeli P, Mateescu MA. Carboxymethyl starch/alginate microspheres containing diamine oxidase for intestinal targeting. Biotechnol Appl Biochem. 2016. https://doi.org/10.1002/bab.1369.
Ebrahimnejad H, Gheisari H, Khan Nazer AH. Pea seedling amine oxidase application: an emerging antihistamine strategy in tuna fish. J Food Process Technol. 2013. https://doi.org/10.4172/2157-7110.1000242.
Leonida M, Belbekhouche S, Adams F, Bijja UK, Choudhary DA, Kumar I. Enzyme nanovehicles: histaminase and catalase delivered in nanoparticulate chitosan. Int J Pharm. 2019. https://doi.org/10.1016/j.ijpharm.2018.12.050.
Snyder SH, Hendley ED. A simple and sensitive fluorescence assay for mono-amine oxidase and diamine oxidase. J Pharmacol Exp Ther. 1968;163:386–92.
Rinaldi A, Floris G, Finazzi-Agro A. Purification and properties of diamine oxidase from Euphorbia latex. Eur J Biochem. 1982. https://doi.org/10.1111/j.1432-1033.1982.tb06888.x.
Federico R, Befani O, Mondovì B, Mulhbacher J, Mateescu MA. Immobilization of plant histaminase for medical applications. Inflamm Res. 2000. https://doi.org/10.1007/PL00000184.
Kivirand K, Rinken T. Purification and properties of amine oxidase from pea seedlings. Proc Estonian Acad Sci Chem. 2007;56:164–71.
Torre R, Costa-Rama E, Lopes P, Nouwsa HPA, Delerue-Matos C. Amperometric enzyme sensor for the rapid determination of histamine. Anal Methods. 2019. https://doi.org/10.1039/c8ay02610f.
Lessof MH, Gant V, Hinuma K, Murphy GM, Dowling RH. Recurrent urticaria and reduced diamine oxidase activity. Clin Exp Allergy. 1990. https://doi.org/10.1111/j.1365-2222.1990.tb02796.x.
Leuschner RG, Heidel M, Hammes WP. Histamine and tyramine degradation by food fermenting microorganisms. Int J Food Microbiol. 1998. https://doi.org/10.1016/S0168-1605(97)00109-8.
Dapkevicius MLNE, Nout MJR, Rombouts FM, Houben JH, Wymenga W. Biogenic amine formation and degradation by potential fish starter microorganisms. Int J Food Microbiol. 2000. https://doi.org/10.1016/S0168-1605(00)00238-5.
Calinescu C, Mondovi B, Federico R, Ispas-Szabo P, Mateescu MA. Carboxymethyl starch: chitosan monolithic matrices containing diamine oxidase and catalase for intestinal delivery. Int J Pharm. 2012. https://doi.org/10.1016/j.ijpharm.2012.02.032.
Ahmadifar SM, Le TC, Marcocci L, Pietrangeli P, Mateescu MA. Zymographic approach to determine the intrinsic enzyme specific activity of diamine oxidase in presence of interfering enzymes. Anal Chim Acta. 2017. https://doi.org/10.1016/j.aca.2017.04.006.
Medda R, Padiglia A, Floris G. Plant copper-amine oxidases. Phytochemistry. 1995. https://doi.org/10.1016/0031-9422(94)00756-J.
Pietrangeli P, Federico R, Mondovì B, Morpurgo L. Substrate specificity of copper-containing plant amine oxidases. J Inorg Biochem. 2007. https://doi.org/10.1016/j.jinorgbio.2007.03.014.
Latorre-Moratalla ML, Bosch-Fusté J, Lavizzari T, Bover-Cid S, Veciana-Nogués MT, Vidal-Carou MC. Validation of an ultra high pressure liquid chromatographic method for the determination of biologically active amines in food. J Chromatogr A. 2009. https://doi.org/10.1016/j.chroma.2009.08.072.
Bouvrette P, Male KB, Luong JHT, Gibbs BF. Amperometric biosensor for diamine using diamine oxidase purified from porcine kidney. Enzym Microb Technol. 1997. https://doi.org/10.1016/S0141-0229(96)00064-6.
Missbichler A, Mayer I, Pongracz C, Gabor F, Komericki P. Supplementation of enteric coated diamine oxidase improves intestinal degradation of food-borne biogenic amines in case of histamine intolerance. Clin Nutr Suppl. 2010. https://doi.org/10.1016/S1744-1161(10)70019-3.
Thompson M, Ellison R, Wood R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl Chem. 2002. https://doi.org/10.1351/pac200274050835.
Horwitz W, Albert R. The Horwitz ratio (HorRat): a useful index of method performance with respect to precision. J AOAC Int. 2006;89:1095–109.
Childs RE, Bardsley WG. The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J. 1975. https://doi.org/10.1042/bj1450093.
Neufeld E, Chayen R. An evaluation of three methods for the measurement of diamine oxidase (DAO) activity in amniotic fluid. Anal Biochem. 1979. https://doi.org/10.1016/0003-2697(79)90601-8.
Komericki P, Klein G, Reider N, Hawranek T, Strimitzer T, Lang R, et al. Histamine intolerance: lack of reproducibility of single symptoms by oral provocation with histamine: a randomised, double-blind, placebo-controlled cross-over study. Wien Klin Wochenschr. 2011. https://doi.org/10.1007/s00508-010-1506-y.
Manzotti G, Breda D, Gioacchino M, Burastero SE. Serum diamine oxidase activity in patients with histamine intolerance. Int J Immunopathol Pharmacol. 2016. https://doi.org/10.1177/0394632015617170.
Yacoub MR, Ramirez GA, Berti A, Mercurio G, Breda D, Saporiti N, et al. Diamine oxidase supplementation in chronic spontaneous urticaria: a randomized, double-blind placebo-controlled study. Int Arch Allergy Immunol. 2018. https://doi.org/10.1159/000488142.
Biegański T, Kusche J, Lorenz W, Hesterberg R, Stahlknecht CD, Feussner KD. Distribution and properties of human intestinal diamine oxidase and its relevance for the histamine catabolism. Biochim Biophys Acta. 1983. https://doi.org/10.1016/0304-4165(83)90092-2.
Küfner MA, Ulrich P, Raithel M, Schwelberger HG. Determination of histamine degradation capacity in extremely small human colon samples. Inflamm Res. 2001. https://doi.org/10.1007/PL00022422.
Petersen J, Raithel M, Schwelberger HG. Characterisation of functional polymorphisms of the human diamine oxidase gene. Inflamm Res. 2005. https://doi.org/10.1007/s00011-004-0426-6.
Acknowledgments
Sònia Sánchez-Pérez is a recipient of a doctoral fellowship from the University of Barcelona (APIF2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Comas-Basté, O., Latorre-Moratalla, M.L., Sánchez-Pérez, S. et al. In vitro determination of diamine oxidase activity in food matrices by an enzymatic assay coupled to UHPLC-FL. Anal Bioanal Chem 411, 7595–7602 (2019). https://doi.org/10.1007/s00216-019-02178-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02178-2