Abstract
Peiyuan Tongnao capsule (PTC) is a prescription medicine of traditional Chinese medicine with the effects of “nourishing the kidney,” “replenishing essence,” “extinguishing wind,” and “opening the meridian”. PTC is also widely used in clinic for the treatment of stroke and chronic cerebral circulation insufficiency. However, the quality control studies of PTC are hitherto quite limited. Here, we aim to fully utilize an advanced chromatography–mass spectrometry hyphenation technique to qualitatively and quantitatively evaluate the quality of PTC. Firstly, a two-dimensional liquid chromatography/quadrupole-Orbitrap mass spectrometry (2D-LC/Q-Orbitrap-MS) approach was established for multicomponent characterization. An offline 2D-LC system fitted with an Xbridge Amide column and an HSS T3 column showed an orthogonality of 0.63 and a theoretical peak capacity of 6930. Eleven fractions of PTC, after hydrophilic interaction chromatography (first dimension), were further analyzed by reversed-phase ultrahigh-performance liquid chromatography/Q-Orbitrap-MS (UHPLC/Q-Orbitrap-MS, second dimension) using a rapid negative/positive switching mode. Consequently, 178 compounds were separated, 96 of which were identified or tentatively characterized. Secondly, co-condition fingerprint analysis of seven constituted herbal medicines of PTC was performed to unveil ten active ingredients (citric acid, rehmannioside D, echinacoside, paeoniflorin, verbascoside, liquiritin, 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside, cinnamic aldehyde, glycyrrhizic acid, and emodin) as the quality markers of PTC. Thirdly, a UHPLC/PRMad (adjusted parallel reaction monitoring) method was established and validated to quantify the ten marker compounds in 14 batches of PTC. To the best of our knowledge, this is the first study to report comprehensive multicomponent characterization, authentication, and quality evaluation of PTC, which could be used to lay the foundation for quality control, biological efficacy research, and further development.

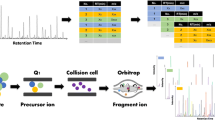
Graphical abstract




Similar content being viewed by others
References
Wang Y, Fu LL, Wang XY, Yang WZ, Wang HD, Zuo TT, et al. Erxian decoction, a Chinese herbal formula, for menopausal syndrome: an updated systematic review. J Ethnopharmacol. 2018;234:8–20. https://doi.org/10.1016/j.jep.2019.01.010.
Yang L, Jiang H, Hou AJ, Guo XY, Man WJ, Yan WL, et al. Simultaneous determination of thirteen Q-markers in raw and processed Tussilago farfara L. by UPLC-QQQ-MS/MS coupled with Chemometrics. Molecules. 2019;24(3):598. https://doi.org/10.3390/molecules24030598.
Wang Q, Tong L, Yao L, Xu L. Fingerprinting of traditional Chinese medicines on the C18-diol mixed-mode column in online or offline two-dimensional liquid chromatography on the single column modes. J Pharm Biomed Anal. 2016;125:205–11. https://doi.org/10.1016/j.jpba.2016.03.033.
Ma XL, Guo XY, Song YL, Qiao LR, Wang WG, Zhao MB, et al. An integrated strategy for global qualitative and quantitative profiling of traditional Chinese medicine formulas: Baoyuan decoction as a case. Sci Rep. 2016;6(1):38379. https://doi.org/10.1038/srep38379.
Shaw LH, Chen WM, Tsai TH. Identification of multiple ingredients for a traditional Chinese medicine preparation (Bu-yang-huan-wu-tang) by liquid chromatography coupled with tandem mass spectrometry. Molecules. 2013;18(9):11281–98. https://doi.org/10.3390/molecules180911281.
Zhong WF, Tong WS, Zhou SS, Yip KM, Li SL, Zhao ZZ, et al. Qualitative and quantitative characterization of secondary metabolites and carbohydrates in Bai-Hu-Tang using ultraperformance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry and ultraperformance liquid chromatography coupled with photodiode array detector. J Food Drug Anal. 2017;25(4):946–59. https://doi.org/10.1016/j.jfda.2016.12.007.
Fairchild JN, Horvath K, Gooding JR, Campagna SR, Guiochon G. Two-dimensional liquid chromatography/mass spectrometry/mass spectrometry separation of water-soluble metabolites. J Chromatogr A. 2010;1217(52):8161–6. https://doi.org/10.1016/j.chroma.2010.10.068.
Qiu S, Yang WZ, Shi XJ, Yao CL, Yang M, Liu X, et al. A green protocol for efficient discovery of novel natural compounds: characterization of new ginsenosides from the stems and leaves of Panax ginseng as a case study. Anal Chim Acta. 2015;893:65–76. https://doi.org/10.1016/j.aca.2015.08.048.
Jia L, Fu LL, Wang XY, Yang WZ, Wang HD, Zuo TT, et al. Systematic profiling of the multicomponents and authentication of Erzhi Pill by UHPLC/Q-Orbitrap-MS oriented rapid polarity-switching data-dependent acquisition and selective monitoring of the chemical markers deduced from fingerprint analysis. Molecules. 2018;23(12):3143. https://doi.org/10.3390/molecules23123143.
Yang WZ, Zhang JX, Yao CL, Qiu S, Chen M, Pan HQ, et al. Method development and application of offline two-dimensional liquid chromatography/quadrupole time-of-flight mass spectrometry-fast data directed analysis for comprehensive characterization of the saponins from Xueshuantong injection. J Pharm Biomed Anal. 2016;128:322–32. https://doi.org/10.1016/j.jpba.2016.05.035.
Wang SY, Qiao LZ, Shi XZ, Hu CX, Kong HW, Xu GW. On-line stop-flow two-dimensional liquid chromatography–mass spectrometry method for the separation and identification of triterpenoid saponins from ginseng extract. Anal Bioanal Chem. 2015;407(1):331–41. https://doi.org/10.1007/s00216-014-8219-4.
Liu YM, Xue XY, Guo ZM, Xu Q, Zhang FF, Liang XM. Novel two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography, an excellent orthogonal system for practical analysis. J Chromatogr A. 2008;1208(1-2):133–40. https://doi.org/10.1016/j.chroma.2008.08.079.
Liu SS, Yang K, Sun ZL, Zheng XF, Bai X, Liu ZY. A novel two-dimensional liquid chromatography system for the simultaneous determination of three monoterpene indole alkaloids in biological matrices. Anal Bioanal Chem. 2019. https://doi.org/10.1007/s00216-019-01859-2.
Fu Q, Guo ZM, Zhang XL, Liu YF, Liang XM. Comprehensive characterization of Stevia Rebaudiana using two-dimensional reversed-phase liquid chromatography/hydrophilic interaction liquid chromatography. J Sep Sci. 2015;35(14):1821–7. https://doi.org/10.1002/jssc.201101103.
Han LF, Wang P, Wang YL, Zhao QY, Zheng F, Dou ZY, et al. Rapid discovery of the potential toxic compounds in Polygonum multiflorum by UHPLC/Q-Orbitrap-MS-based metabolomics and correlation analysis. Front Pharmacol. 2019. https://doi.org/10.3389/fphar.2019.00329.
Liu CY, Ma RF, Wang LL, Zhu RY, Liu HX, Guo Y, et al. Rehmanniae Radix, in osteoporosis: a review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J Ethnopharmacol. 2017;198:351–62. https://doi.org/10.1016/j.jep.2017.01.021.
Han LF, Yiadom MB, Liu EW, Zhang Y, Li W, Song XB. Structural characterisation and identification of phenylethanoid glycosides from Cistanches deserticola Y.C. Ma by UHPLC/ESI–QTOF–MS/MS. Phytochem Anal. 2012;23(6):668–76. https://doi.org/10.1002/pca.2371.
Zhao YL, Jia RH, Qiao Y, Wang DY. Glycyrrhizic acid, active component from Glycyrrhizae radix, prevents toxicity of graphene oxide by influencing functions of microRNAs in nematode Caenorhabditis elegans. Nanomedicine: NBM. 2016;12(3):735–44. https://doi.org/10.1016/j.nano.2015.10.008.
Li ZQ, Liu J, Zhang DQ, Du X, Han LF, Lv CX, et al. Nuciferine and paeoniflorin can be quality markers of Tangzhiqing tablet, a Chinese traditional patent medicine, based on the qualitative, quantitative and dose-exposure-response analysis. Phytomedicine. 2018;44:155–63. https://doi.org/10.1016/j.phymed.2018.02.006.
Jurikova T, Sochor J, Rop O, Mlcek J, Balla S, Szekeres L, et al. Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules. 2012;17(12):14490–509. https://doi.org/10.3390/molecules171214490.
Zhang HJ, Sydara K, Tan GT, Ma CY, Southavong B, Soejarto DD, et al. Bioactive constituents from Asparagus cochinchinensis. J Nat Prod. 2004;67(2):194–200. https://doi.org/10.1021/np030370b.
Zhang JX, Yang WZ, Li SR, Yao S, Qi P, Yang Z, et al. An intelligentized strategy for endogenous small molecules characterization and quality evaluation of earthworm from two geographic origins by ultra-high performance HILIC/QTOF MSE and Progenesis QI. Anal Bioanal Chem. 2016;408(14):3881–90. https://doi.org/10.1007/s00216-016-9482-3.
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, part 1. Beijing: Chemical Industry; 2015.
Yang WZ, Zhang YB, Wu WY, Huang LQ, Guo DA, Liu CX. Approaches to establish Q-markers for the quality standards of traditional Chinese medicines. Acta Pharm Sinica B. 2017;7(4):439–46. https://doi.org/10.1016/j.apsb.2017.04.012.
Kang TG, Dou DQ, Xu L. Establishment of a quality marker (Q-marker) system for Chinese herbal medicines using burdock as an example. Phytomedicine. 2019;54:339–46. https://doi.org/10.1016/j.phymed.2018.04.005.
Gao X, Du X, An LJ, Wang YY, Wang LL, Wu ZG, et al. Wilforine, the Q-marker and PK-maker of tripterygium glycosides tablet: based on preparation quantitative analysis and PK-PD study. Phytomedicine. 2019;54:357–64. https://doi.org/10.1016/j.phymed.2018.03.031.
Duan SN, Qi W, Zhang SW, Huang KK, Yuan D. Simultaneous quantification combined with multivariate statistical metabolites and carbohydrates in Bai-Hu-Tang using ultraperformance liquid chromatography coupled with analysis of multiple chemical maekers of Wu Ji Bai Feng Pill by UHPLC-MS/MS. J Food Drug Anal. 2019;27(1):275–83. https://doi.org/10.1016/j.jfda.2018.10.004.
Nie Y, Yao WF. A comprehensive quality evaluation method based on C30-HPLC and an analytic hierarchy process for the Chinese herbal formula, Erzhiwan. Molecules. 2018;23(8):2045. https://doi.org/10.3390/molecules23082045.
Yi L, Qi LW, Li P, Ma YH, Luo YJ, Li HY. Simultaneous determination of bioactive constituents in Danggui Buxue Tang for quality control by HPLC coupled with a diode array detector, an evaporative light scattering detector and mass spectrometry. Anal Bioanal Chem. 2007;389(2):571–80. https://doi.org/10.1007/s00216-007-1431-8.
Chen CH, Liu ZX, Zou LS, Liu XH, Chai C, Zhao H, et al. Quality evaluation of Apocyni Veneti Folium from different habitats and commercial herbs based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Molecules. 2018;23(3):573. https://doi.org/10.3390/molecules23030573.
Yan Y, Song QQ, Chen XJ, Li J, Li P, Wang YT, et al. Simultaneous determination of components with wide polarity and content ranges in, Cistanche tubulosa, using serially coupled reverse phase-hydrophilic interaction chromatography-tandem mass spectrometry. J Chromatogr A. 2017;1501:39–50. https://doi.org/10.1016/j.chroma.2017.04.034.
Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol Cell Proteomics. 2012;11(11):1475–88. https://doi.org/10.1074/mcp.O112.020131.
Chen SM, Yang C, Downs ML. Detection of six commercially processed soy ingredients in an incurred food matrix using parallel reaction monitoring. J Proteome Res. 2019;18(3):995–1005. https://doi.org/10.1021/acs.jproteome.8b00689.
Kim KH, Park GW, Jeong JE, Ji ES, An HJ, Kim JY, et al. Parallel reaction monitoring with multiplex immunoprecipitation of N-glycoproteins in human serum for detection of hepatocellular carcinoma. Anal Bioanal Chem. 2019;411(14):3009–19. https://doi.org/10.1007/s00216-019-01775-5.
Abdallah MAE, Nguyen KH, Ebele AJ, Atia NN, Ali HRH, Harrad S. A single run, rapid polarity switching method for determination of 30 pharmaceuticals and personal care products in waste water using Q-Exactive Orbitrap high resolution accurate mass spectrometry. J Chromatogr A. 2018;1588:68–76. https://doi.org/10.1016/j.chroma.2018.12.033.
Tague ED, Woodall BM, Harp JR, Farmer AT, Fozo EM, Campagna SR. Expanding lipidomics coverage: efective ultra performance liquid chromatography-high resolution mass spectrometer methods for detection and quantitation of cardiolipin, phosphatidylglycerol, and lysyl-phosphatidylglycerol. Metabolomics. 2019. https://doi.org/10.1007/s11306-019-1512-7.
Camenzuli M, Schoenmakers PJ. A new measure of orthogonality for multi-dimensional chromatography. Anal Chim Acta. 2014;838:93–101. https://doi.org/10.1016/j.aca.2014.05.048.
Nie CH, Zhang FG, Ma XW, Guo R, Zhou SP, Zhao LB, et al. Determination of quality markers of Xuezhiling tablet for hyperlipidemia treatment. Phytomedicine. 2018;44:231–8. https://doi.org/10.1016/j.phymed.2018.03.004.
Li F, Yang XL, Yang YN, Guo CR, Zhang CF, Yang ZL, et al. Antiosteoporotic activity of echinacoside in ovariectomized rats. Phytomedicine. 2013;20(6):549–57. https://doi.org/10.1016/j.phymed.2013.01.001.
Acknowledgements
This study was supported by National Key R&D Program of China (2018YFC1704500) and Tianjin Committee of Science and Technology, China (18JCYBJC94700). Many thanks for sampling support from Henan Lingrui Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 3853 kb)
Rights and permissions
About this article
Cite this article
Wang, C., Feng, K., Fu, Z. et al. Systematic quality evaluation of Peiyuan Tongnao capsule by offline two-dimensional liquid chromatography/quadrupole-Orbitrap mass spectrometry and adjusted parallel reaction monitoring of quality markers. Anal Bioanal Chem 411, 7747–7760 (2019). https://doi.org/10.1007/s00216-019-02119-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02119-z