Abstract
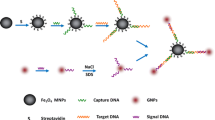
An immunomagnetic optical probe based on a core/shell magnetic nanoparticle–quantum dot was fabricated for detection of Streptococcus agalactiae, the causative agent of pneumonia and meningitis in newborns. The silica-coated magnetic nanoparticles conjugated with anti-S. agalactiae monoclonal antibody provided high specificity for pre-enrichment of bacteria from biological samples with a complex matrix such as milk. Compared with conventional methods such as culture and molecular techniques, the combination of fluorescent quantum dot and magnetic nanoparticle enhanced the sensitivity and speed of bacterial identification. The bio-functionalized fluorescent-magnetic nanoparticles were characterized by TEM, SEM, VSM, XRD, DLS, and FTIR and applied to the detection of S. agalactiae with a limit of 10 and 102 CFU/mL in PBS and milk, respectively. This immunomagnetic optical probe can be used for rapid isolation, sensitive, and specific detection of targeted bacteria without any treatment in clinical and animal samples in the presence of other infectious agents.





Similar content being viewed by others
Abbreviations
- Ab:
-
Antibody
- FMNP-Ab:
-
Antibody-conjugated FMNPs
- FMNPs:
-
Fluorescent-magnetic nanocomposite
- MNPs:
-
Magnetic nanoparticles
- QDs:
-
Quantum dots
References
Dye C. After 2015: infectious diseases in a new era of health and development. Philos Trans R Soc B. 2014;369(1645):20130426.
Anthony BF, Okada DM. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28(1):355–69.
Mweu MM, Nielsen SS, Halasa T, Toft N. Annual incidence, prevalence and transmission characteristics of Streptococcus agalactiae in Danish dairy herds. Prev Vet Med. 2012;106(3-4):244–50.
Lyhs U, Kulkas L, Katholm J, Waller KP, Saha K, Tomusk RJ, et al. Streptococcus agalactiae serotype IV in humans and cattle, northern Europe. Emerging Infect Dis. 2016;22(12):2097.
Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention; 2010.
Smolinski MS, Hamburg MA, Lederberg J. Pathogen discovery, detection, and diagnostics . Microbial threats to health: emergence, detection, and response. Institute of Medicine (US) Committee on Emerging Microbial Threats to Health in the 21st Century; Washington (DC): National Academies Press (US); 2003.
Behera T, Swain P, Mohapatra D. Virulence determination of bacterial isolates through culture in India ink including broth. J Microbiol Antimicrob. 2013;5(8):87–90.
Sousa AM, Pereira MO. A prospect of current microbial diagnosis methods. In: Mendez-Vilas A, editor. Microbial pathogens and strategies for combating them: science, technology and education, vol. 3. Badajoz: Formatex; 2013. p. 1429–38.
Wallet F, Nseir S, Baumann L, Herwegh S, Sendid B, Boulo M, et al. Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin Microbiol Infect. 2010;16(6):774–9.
Weile J, Knabbe C. Current applications and future trends of molecular diagnostics in clinical bacteriology. Anal Bioanal Chem. 2009;394(3):731–42.
Ni P-X, Ding X, Zhang Y-X, Yao X, Sun R-X, Wang P, et al. Rapid detection and identification of infectious pathogens based on high-throughput sequencing. Chin Med J. 2015;128(7):877.
Boardman AK, Wong WS, Premasiri WR, Ziegler LD, Lee JC, Miljkovic M, et al. Rapid detection of bacteria from blood with surface-enhanced Raman spectroscopy. Anal Chem. 2016;88(16):8026–35.
Stevens KA, Jaykus L-A. Bacterial separation and concentration from complex sample matrices: a review. Crit Rev Microbiol. 2004;30(1):7–24.
Palchetti I, Mascini M. Electroanalytical biosensors and their potential for food pathogen and toxin detection. Anal Bioanal Chem. 2008;391(2):455–71.
Arruebo M, Fernández-Pacheco R, Velasco B, Marquina C, Arbiol J, Irusta S, et al. Antibody-functionalized hybrid superparamagnetic nanoparticles. Adv Funct Mater. 2007;17(9):1473–9.
Cudjoe KS, Hagtvedt T, Dainty R. Immunomagnetic separation of Salmonella from foods and their detection using immunomagnetic particle (IMP)-ELISA. Int J Food Microbiol. 1995;27(1):11–25.
Fu Z, Rogelj S, Kieft TL. Rapid detection of Escherichia coli O157: H7 by immunomagnetic separation and real-time PCR. Int J Food Microbiol. 2005;99(1):47–57.
Fontaine M, Guillot E. An immunomagnetic separation–real-time PCR method for quantification of Cryptosporidium parvum in water samples. J Microbiol Methods. 2003;54(1):29–36.
Dwivedi HP, Smiley RD, Jaykus L-A. Selection of DNA aptamers for capture and detection of Salmonella typhimurium using a whole-cell SELEX approach in conjunction with cell sorting. Appl Microbiol Biotechnol. 2013;97(8):3677–86.
Poshtiban S, Javed MA, Arutyunov D, Singh A, Banting G, Szymanski CM, et al. Phage receptor binding protein-based magnetic enrichment method as an aid for real time PCR detection of foodborne bacteria. Analyst. 2013;138(19):5619–26.
Wang Y, Salazar JK. Culture-independent rapid detection methods for bacterial pathogens and toxins in food matrices. Compr Rev Food Sci Food Saf. 2016;15(1):183–205.
Jeníková G, Pazlarová J, Demnerová K. Detection of Salmonella in food samples by the combination of immunomagnetic separation and PCR assay. Int Microbiol. 2010;3(4):225–9.
Mansfield L, Forsythe S. The detection of Salmonella using a combined immunomagnetic separation and ELISA end-detection procedure. Lett Appl Microbiol. 2000;31(4):279–83.
Hibi K, Abe A, Ohashi E, Mitsubayashi K, Ushio H, Hayashi T, et al. Combination of immunomagnetic separation with flow cytometry for detection of Listeria monocytogenes. Anal Chim Acta. 2006;573:158–63.
Varshney M, Li Y, Nanapanneni R, Johnson MG, Griffis CL. A chemiluminescence biosensor coupled with immunomagnetic separation for rapid detection of Salmonella typhimurium. J Rapid Methods Autom Microbiol. 2003;11(2):111–31.
Lazcka O, Campo F, Munoz FX. Pathogen detection: a perspective of traditional methods and biosensors. Biosens Bioelectron. 2007;22(7):1205–17.
Nayak M, Kotian A, Marathe S, Chakravortty D. Detection of microorganisms using biosensors—a smarter way towards detection techniques. Biosens Bioelectron. 2009;25(4):661–7.
Damborský P, Švitel J, Katrlík J. Optical biosensors. Essays Biochem. 2016;60(1):91–100.
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4(6):435.
Samir TM, Mansour MM, Kazmierczak SC, Azzazy HM. Quantum dots: heralding a brighter future for clinical diagnostics. Nanomedicine. 2012;7(11):1755–69.
McMillan J, Batrakova E, Gendelman HE. Cell delivery of therapeutic nanoparticles. Prog Mol Biol Transl Sci. 2011;104:563.
Bentolila LA, Ebenstein Y, Weiss S. Quantum dots for in vivo small-animal imaging. J Nucl Med. 2009;50(4):493–6.
Peng C-W, Li Y. Application of quantum dots-based biotechnology in cancer diagnosis: current status and future perspectives. J Nanomater. 2010. https://doi.org/10.1155/2010/676839.
Rosenthal SJ, Chang JC, Kovtun O, McBride JR, Tomlinson ID. Biocompatible quantum dots for biological applications. Chem Biol. 2011;18(1):10–24.
Li J, Zhu J-J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst. 2013;138(9):2506–15.
Di Corato R, Bigall NC, Ragusa A, Dorfs D, Genovese A, Marotta R, et al. Multifunctional nanobeads based on quantum dots and magnetic nanoparticles: synthesis and cancer cell targeting and sorting. ACS Nano. 2011;5(2):1109–21.
Yaohua H, Chengcheng W, Bing B, Mintong L, Wang R, Li Y. Detection of Staphylococcus aureus using quantum dots as fluorescence labels. Int J Agric Biol Eng. 2014;7(1):77–83.
You X, He R, Gao F, Shao J, Pan B, Cui D. Hydrophilic high-luminescent magnetic nanocomposites. Nanotechnology. 2007;18(3):035701.
Byrne B, Stack E, Gilmartin N, O'Kennedy R. Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors. 2009;9(6):4407–45.
Kaittanis C, Santra S, Perez J. Emerging nanotechnology-based strategies for the identification of microbial pathogenesis. Adv Drug Delivery Rev. 2010;62(4):408–23.
Jamdagni P, Khatri P, Rana J. Nanoparticles based DNA conjugates for detection of pathogenic microorganisms. Int Nano Lett. 2016;6(3):139–46.
Singh G, Manohar M, Adegoke AA, Stenström TA, Shanker R. Novel aptamer-linked nanoconjugate approach for detection of waterborne bacterial pathogens: an update. J Nanopart Res. 2017;19(1):4.
Ma J, Fan Q, Wang L, Jia N, Gu Z, Shen H. Synthesis of magnetic and fluorescent bifunctional nanocomposites and their applications in detection of lung cancer cells in humans. Talanta. 2010;81(4):1162–9.
Xie H-Y, Xie M, Zhang Z-L, Long Y-M, Liu X, Tang M-L, et al. Wheat germ agglutinin-modified trifunctional nanospheres for cell recognition. Bioconjugate Chem. 2007;18(6):1749–55.
Sun P, Zhang H, Liu C, Fang J, Wang M, Chen J, et al. Preparation and characterization of Fe3O4/CdTe magnetic/fluorescent nanocomposites and their applications in immuno-labeling and fluorescent imaging of cancer cells. Langmuir. 2009;26(2):1278–84.
Xu Y, Karmakar A, Wang D, Mahmood MW, Watanabe F, Zhang Y, et al. Multifunctional Fe3O4 cored magnetic-quantum dot fluorescent nanocomposites for RF nanohyperthermia of cancer cells. J Phys Chem C. 2010;114(11):5020–6.
Yang D, Hu J, Fu S. Controlled synthesis of magnetite−silica nanocomposites via a seeded sol−gel approach. J Phys Chem C. 2009;113(18):7646–51.
Binaymotlagh R, Haghighi FH, Aboutalebi F, Mirahmadi-Zare SZ, Hadadzadeh H, Nasr-Esfahani M-H. Selective chemotherapy and imaging of colorectal and breast cancer cells by a modified MUC-1 aptamer conjugated to a poly(ethylene glycol)-dimethacrylate coated Fe3O4–AuNCs nanocomposite. New J Chem. 2019;43(1):238–48.
Shoghi E, Mirahmadi-Zare SZ, Ghasemi R, Asghari M, Poorebrahim M, Nasr-Esfahani M-H. Nanosized aptameric cavities imprinted on the surface of magnetic nanoparticles for high-throughput protein recognition. Microchim Acta. 2018;185(4):241.
Silva FO, Carvalho MS, Mendonça R, Macedo WA, Balzuweit K, Reiss P, et al. Effect of surface ligands on the optical properties of aqueous soluble CdTe quantum dots. Nanoscale Res Lett. 2012;7:536.
Wang C, Wang J, Li M, Qu X, Zhang K, Rong Z, et al. A rapid SERS method for label-free bacteria detection using polyethylenimine-modified Au-coated magnetic microspheres and Au@Ag nanoparticles. Analyst. 2016;141(22):6226–38.
HuiáShin H, JoonáCha H. A facile and sensitive detection of pathogenic bacteria using magnetic nanoparticles and optical nanocrystal probes. Analyst. 2012;137(16):3609–12.
Bai Y, Song M, Cui Y, Shi C, Wang D, Paoli GC, et al. A rapid method for the detection of foodborne pathogens by extraction of a trace amount of DNA from raw milk based on amino-modified silica-coated magnetic nanoparticles and polymerase chain reaction. Anal Chim Acta. 2013;787:93–101.
Gao J, Li L, Ho PL, Mak GC, Gu H, Xu B. Combining fluorescent probes and biofunctional magnetic nanoparticles for rapid detection of bacteria in human blood. Adv Mater. 2006;18(23):3145–8.
Cao C, Gontard LC, Thuy Tram LL, Wolff A, Bang DD. Dual enlargement of gold nanoparticles: from mechanism to scanometric detection of pathogenic bacteria. Small. 2011;7(12):1701–8.
Dogan Ü, Kasap E, Cetin D, Suludere Z, Boyaci IH, Türkyılmaz C, et al. Rapid detection of bacteria based on homogenous immunoassay using chitosan modified quantum dots. Sens Actuators B. 2016;233:369–78.
Xue L, Zheng L, Zhang H, Jin X, Lin J. An ultrasensitive fluorescent biosensor using high gradient magnetic separation and quantum dots for fast detection of foodborne pathogenic bacteria. Sens Actuators B. 2018;265:318–25.
Acknowledgment
We are grateful for the financial support from the Iran National Institute for Medical Research Development (NIMAD) (Grant No. 940990).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghasemi, R., Mirahmadi-zare, S.Z., Nasr-Esfahani, M.H. et al. Optical biosensing of Streptococcus agalactiae based on core/shell magnetic nanoparticle-quantum dot. Anal Bioanal Chem 411, 6733–6743 (2019). https://doi.org/10.1007/s00216-019-02046-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02046-z