Abstract
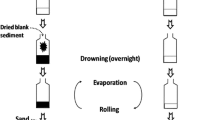
Ecotoxicological screening of surface waters can involve multiple analyses using multiple bioassay and chemical analytical methods that require enriched samples to reach low concentrations. Such broad screening of the same sample necessitates sufficient sample volume—typically several liters—to produce a sufficient amount of enriched sample. Often, this is achieved by performing parallel solid-phase extractions (SPE) where extracts are combined into a pool—this is a laborious process. In this study, we first validated our existing SPE method for the chemical recovery of an extended set of compounds. We spiked four estrogenic compounds and 11 herbicides to samples from independent rivers (1 L) and wastewater treatment plant effluents (0.5 L). Then, we investigated the effect of increased sample loading of the SPE cartridges on both chemical and biological recoveries by comparing the validated volumes with four times larger sample volumes (i.e., 4 L river water and 2 L effluent). Samples were analyzed by LC-MS/MS and three bioassays: an estrogen receptor transactivation assay (ERα-CALUX), the combined algae test, and a bacterial bioluminescence inhibition assay. Our existing SPE method was found to be suitable for enriching the extended set of estrogens and herbicides in river water and effluents with near to perfect chemical recoveries (~ 100%), except for the herbicide metribuzin (46 ± 19%). In the large volume river and effluent samples, the biological activities and concentrations of the spiked compounds were between 87 and 104% of those measured with the lower sample loading, which is adequate. In addition, the ratio between the large and original volume SPE method for the non-target endpoint (bacterial bioluminescence inhibition) was acceptable (on average 82 ± 9%). Results indicate that our current water extraction method can be applied to up to four times larger sample volumes, resulting in four times more extract volumes, without significant reductions in recoveries for the tested estrogens and herbicides.

ᅟ





Similar content being viewed by others
References
Escher BI, Bramaz N, Quayle P, Rutishauser S, Vermeirssen ELM. Monitoring of the ecotoxicological hazard potential by polar organic micropollutants in sewage treatment plants and surface waters using a mode-of-action based test battery. J Environ Monit. 2008;10(5):622–31.
Kienle C, Vermeirssen E, Kunz P, Werner I. Grobbeurteilung der Wasserqualität von abwasserbelasteten Gewässern anhand von ökotoxikologischen Biotests. Studie im Auftrag des BAFU. Dübendorf: Schweizerisches Zentrum für angewandte Oekotoxikologie Eawag-EPFL; 2015.
Rvd O, Sileno G, Suárez-Muñoz M, Nguyen MT, Besselink H, Brouwer A. SIMONI (Smart Integrated Monitoring) as a novel bioanalytical strategy for water quality assessment: Part I–model design and effect-based trigger values. Environ Toxicol Chem. 2017;36(9):2385–99.
Wernersson A-S, Carere M, Maggi C, Tusil P, Soldan P, James A, et al. The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ Sci Eur. 2015;27(1):7.
Durand AM, Rotteveel S, Collombon MT, van der Grinten E, Maas JL, Verweij W. Toxicity measurements in concentrated water samples. Evaluation and validation. 2009. Contract No.: Report 607013010/2009, Centre for Water Management Report 2009.003.
Neale PA, Munz NA, Aїt-Aїssa S, Altenburger R, Brion F, Busch W, et al. Integrating chemical analysis and bioanalysis to evaluate the contribution of wastewater effluent on the micropollutant burden in small streams. Sci Total Environ. 2017;576(Supplement C):785–95.
Välitalo P, Massei R, Heiskanen I, Behnisch P, Brack W, Tindall AJ, et al. Effect-based assessment of toxicity removal during wastewater treatment. Water Res. 2017;126:153–63.
Maruya KA, Dodder NG, Mehinto AC, Denslow ND, Schlenk D, Snyder SA, et al. A tiered, integrated biological and chemical monitoring framework for contaminants of emerging concern in aquatic ecosystems. Integr Environ Assess Manag. 2016;12(3):540–7.
Könemann S, Kase R, Simon E, Swart K, Buchinger S, Schlüsener M, et al. Effect-based and chemical analytical methods to monitor estrogens under the European Water Framework Directive. TrAC Trends Anal Chem. 2018;102:225–35
Tang JYM, Busetti F, Charrois JWA, Escher BI. Which chemicals drive biological effects in wastewater and recycled water? Water Res. 2014;60:289–99.
Creusot N, Aït-Aïssa S, Tapie N, Pardon P, Brion F, Sanchez W, et al. Identification of synthetic steroids in river water downstream from pharmaceutical manufacture discharges based on a bioanalytical approach and passive sampling. Environ Sci Technol. 2014;48(7):3649–57.
Furuichi T, Kannan K, Giesy JP, Masunaga S. Contribution of known endocrine disrupting substances to the estrogenic activity in Tama River water samples from Japan using instrumental analysis and in vitro reporter gene assay. Water Res. 2004;38(20):4491–501.
Muschket M, Di Paolo C, Tindall AJ, Touak G, Phan A, Krauss M, et al. Identification of unknown antiandrogenic compounds in surface waters by effect-directed analysis (EDA) using a parallel fractionation approach. Environ Sci Technol. 2018;52(1):288–97.
Barrek S, Cren-Olivé C, Wiest L, Baudot R, Arnaudguilhem C, Grenier-Loustalot M-F. Multi-residue analysis and ultra-trace quantification of 36 priority substances from the European Water Framework Directive by GC–MS and LC-FLD-MS/MS in surface waters. Talanta. 2009;79(3):712–22.
Neale PA, Brack W, Ait-Aissa S, Busch W, Hollender J, Krauss M, et al. Solid-phase extraction as sample preparation of water samples for cell-based and other in vitro bioassays. Environ Sci Process Impacts. 2018;20(3):493–504.
Escher BI, Leusch FDL. Bioanalytical tools in water quality assessment. London: IWA Publishing; 2012.
Ballesteros-Gómez A, Rubio S. Recent advances in environmental analysis. Anal Chem. 2011;83(12):4579–613.
Escher BI, Bramaz N, Maurer M, Richter M, Sutter D, von Kanel C, et al. Screening test battery for pharmaceuticals in urine and wastewater. Environ Toxicol Chem. 2005;24(3):750–8.
Simon E, Lamoree MH, Hamers T, de Boer J. Challenges in effect-directed analysis with a focus on biological samples. TrAc Trends Anal Chem. 2015;67:179–91.
Krauss M, Singer H, Hollender J. LC–high resolution MS in environmental analysis: from target screening to the identification of unknowns. Anal Bioanal Chem. 2010;397(3):943–51.
Andrade-Eiroa A, Canle M, Leroy-Cancellieri V, Cerda V. Solid-phase extraction of organic compounds: a critical review (part I). TrAc Trends Anal Chem. 2016;80:641–54.
Andrade-Eiroa A, Canle M, Leroy-Cancellieri V, Cerda V. Solid-phase extraction of organic compounds: a critical review. TrAc Trends Anal Chem. 2016;80:655–67.
Schindler Wildhaber Y, Mestankova H, Schärer M, Schirmer K, Salhi E, von Gunten U. Novel test procedure to evaluate the treatability of wastewater with ozone. Water Res. 2015;75(Supplement C):324–35.
Escher BI, Allinson M, Altenburger R, Bain PA, Balaguer P, Busch W, et al. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ Sci Technol. 2014;48(3):1940–56.
Burkhardt-Holm P, Segner H, Burki R, Peter A, Schubert S, Suter MJ-F, et al. Estrogenic endocrine disruption in Switzerland: assessment of fish exposure and effects. Chimica. 2008;62:376–82.
Trachsel M. Consensus platform “Endocrine Disruptors in Waste Water and the Aquatic Environment”. Final document January 2008. http://www.snf.ch/SiteCollectionDocuments/nfp/nfp50/nfp50_schlussdoku_cp_wasser_e.pdf
Margot J, Kienle C, Magnet A, Weil M, Rossi L, de Alencastro LF, et al. Treatment of micropollutants in municipal wastewater: ozone or powdered activated carbon? Sci Total Environ. 2013;461–462(Supplement C):480–98.
Gälli R, Ort C, Schärer M. Mikroverunreinigungen in den Gewässern. Bewertung und Reduktion der Schadstoffbelastung aus der Siedlungsentwässerung. UmweltWissen Nr. 0917. Bundesamt für Umwelt, Bern. 2009.
Rutishauser BV, Pesonen M, Escher BI, Ackermann GE, Aerni HR, Suter MJ-F, et al. Comparative analysis of estrogenic activity in sewage treatment plant effluents involving three in vitro assays and chemical analysis of steroids. Environ Toxicol Chem. 2004;23(4):857–64.
Vethaak AD, Lahr J, Schrap SM, Belfroid AC, Rijs GBJ, Gerritsen A, et al. An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of The Netherlands. Chemosphere. 2005;59(4):511–24.
EU Commission Implementing Decision 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Official Journal of the European Union. Notified under document C(2015) 1756; 2015
Leusch FDL, de Jager C, Levi Y, Lim R, Puijker L, Sacher F, et al. Comparison of five in vitro bioassays to measure estrogenic activity in environmental waters. Environ Sci Technol. 2010;44(10):3853–60.
Kinnberg K. Evaluation of in vitro assays for determination of estrogenic activity in the environment. Working Report No. 43. Danish Environmental Protection Agency: Denmark; 2003.
Bistan M, Podgorelec M, Logar RM, Tisler T. Yeast estrogen screen assay as a tool for detecting estrogenic activity in water bodies. Food Technol Biotechnol. 2012;4(50):427–33.
Krein A, Pailler J-Y, Guignard C, Gutleb AC, Hoffmann L, Meyer B, et al. Determination of estrogen activity in river waters and wastewater in Luxembourg by chemical analysis and the yeast estrogen screen assay. Environ Pollut. 2012;1(2):86–96.
Murk AJ, Legler J, van Lipzig MMH, Meerman JHN, Belfroid AC, Spenkelink A, et al. Detection of estrogenic potency in wastewater and surface water with three in vitro bioassays. Environ Toxicol Chem. 2002;21(1):16–23.
Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol. 1998;32(11):1549–58.
Hettwer K, Jähne M, Frost K, Giersberg M, Kunze G, Trimborn M, et al. Validation of Arxula yeast estrogen screen assay for detection of estrogenic activity in water samples: results of an international interlaboratory study. Sci Total Environ. 2018;621:612–25.
Kunz PY, Simon E, Creusot N, Jayasinghe BS, Kienle C, Maletz S, et al. Effect-based tools for monitoring estrogenic mixtures: evaluation of five in vitro bioassays. Water Res. 2017;110(Supplement C):378–88.
ISO 19040-1-3:2018. International Organization for Standardization (ISO). Standards Catalogue. Water quality - determination of the estrogenic potential of water and waste water - Part 1–3. ISO/TC 147/SC 5 Biological methods. Geneva, Switzerland; 2018.
Chèvre N, Loepfe C, Singer H, Stamm C, Fenner K, Escher BI. Including mixtures in the determination of water quality criteria for herbicides in surface water. Environ Sci Technol. 2006;40(2):426–35.
Moschet C, Wittmer I, Simovic J, Junghans M, Piazzoli A, Singer H, et al. How a complete pesticide screening changes the assessment of surface water quality 2014.
EU Commission Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council; 2008.
Escher BI, Rutishauser S. The combined algae test- a new routine 96-well-plate biotest for simultaneously assessing the photosynthesis inhibition and effect on growth in green algae. Internal Report: Eawag, Dübendorf, Switzerland; 2007.
Schreiber U, Quayle P, Schmidt S, Escher BI, Mueller JF. Methodology and evaluation of a highly sensitive algae toxicity test based on multiwell chlorophyll fluorescence imaging. Biosens Bioelectron. 2007;22(11):2554–63.
Sjollema SB, van Beusekom SA, van der Geest HG, Booij P, de Zwart D, Vethaak AD, et al. Laboratory algal bioassays using PAM fluorometry: effects of test conditions on the determination of herbicide and field sample toxicity. Environ Toxicol Chem. 2014;33(5):1017–22.
Ternes TA, Stumpf M, Mueller J, Haberer K, Wilken RD, Servos M. Behavior and occurrence of estrogens in municipal sewage treatment plants - I. Investigations in Germany, Canada and Brazil. Sci Total Environ. 1999;225:81-90.
ISO 11348-3:1998. International Organization for Standardization (ISO). Standard Catalogue. Water quality–determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (luminescent bacteria test) - Part 3: Method using freeze-dried bacteria. Geneva, Switzerland; 1998.
Escher BI, Bramaz N, Mueller JF, Quayle P, Rutishauser S, Vermeirssen ELM. Toxic equivalent concentrations (TEQs) for baseline toxicity and specific modes of action as a tool to improve interpretation of ecotoxicity testing of environmental samples. J Environ Monit. 2008;10(5):612–21.
Landgraf M, Claudino da Silva S, Rezende M. Mechanism of metribuzin herbicide sorption by humic acid samples from peat and vermicompost. Anal. Chim. Acta. 1998;368:155–64.
Papadakis EN, Papadopoulou-Mourkidou E. Determination of metribuzin and major conversion products in soils by microwave-assisted water extraction followed by liquid chromatographic analysis of extracts. J. Chromatogr. A 2002;962:9–20.
Johnson WE, Fendinger NJ, Plimmer JR. Solid-phase extraction of pesticides from water: possible interferences from dissolved organic material. Anal Cham. 1991;63(15):1510–3.
Hela DG, Sakellarides TM, Konstantinou IK, Albanis TA. Influence of salinity and dissolved humic acids on pesticides extraction from water using solid-phase extraction disks. Int J Environ Anal Chem. 1997;68(1):69–82.
Wells MJM, Riemer DD, Wells-Knecht MC. Development and optimization of a solid-phase extraction scheme for determination of the pesticides metribuzin, atrazine, metolachlor and esfenvalerate in agricultural runoff water. J Chromatogr A. 1994;659(2):337–48.
Sabik H, Jeannot R, Rondeau B. Multiresidue methods using solid-phase extraction techniques for monitoring priority pesticides, including triazines and degradation products, in ground and surface waters. J Chromatogr A. 2000;885(1):217–36.
US EPA. Method 3535A solid-phase extraction (SPE). 2007. https://www.epa.gov/sites/production/files/2015-12/documents/3535a.pdf
Author information
Authors and Affiliations
Contributions
Eszter Simon, Inge Werner, and Etiënne Vermeirssen conceived the study. Eszter Simon, Andrea Schifferli, Thomas Bucher, Daniel Olbrich, and Etiënne Vermeirssen planned the experiments. Andrea Schifferli, Thomas Bucher, and Daniel Olbrich carried out the sampling, the sample preparation and the bioassay and chemical analyses. Andrea Schifferli, Thomas Bucher, Daniel Olbrich, and Eszter Simon performed the calculations. Eszter Simon took the lead in writing the manuscript. All authors contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 726 kb)
Rights and permissions
About this article
Cite this article
Simon, E., Schifferli, A., Bucher, T.B. et al. Solid-phase extraction of estrogens and herbicides from environmental waters for bioassay analysis—effects of sample volume on recoveries. Anal Bioanal Chem 411, 2057–2069 (2019). https://doi.org/10.1007/s00216-019-01628-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01628-1