Abstract
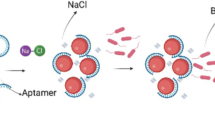
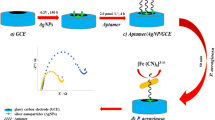
Despite of various advancements in biosensing, a rapid, accurate, and on-site detection of a bacterial pathogen is a real challenge due to the lack of appropriate diagnostic platforms. To address this unmet need, we herein report an aptamer-mediated tunable NanoZyme sensor for the detection of Pseudomonas aeruginosa, an infectious bacterial pathogen. Our approach exploits the inherent peroxidase-like NanoZyme activity of gold nanoparticles (GNPs) in combination with high affinity and specificity of a Pseudomonas aeruginosa–specific aptamer (F23). The presence of aptamer inhibits the inherent peroxidase-like activity of GNPs by simple adsorption on to the surface of GNPs. However, in the presence of cognate target (P. aeruginosa), owing to the high affinity for P. aeruginosa, the aptamer leaves the GNP surface, allowing GNPs to resume their peroxidase-like activity, resulting in oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB). As TMB is an electrochemically active species, we have been able to translate the NanoZyme-based method into an ultrasensitive electrochemical assay using disposable carbon screen-printed electrode. This approach is highly sensitive and allows us to rapidly detect P. aeruginosa with a low-end detection limit of ~ 60 CFU/mL in water within 10 min. This generic aptamer-NanoZyme-based electrochemical sensing strategy may, in principle, be applicable for the detection of various other bacterial pathogens.




Similar content being viewed by others
References
Tang Y, Ali Z, Zou J, Jin G, Zhu J, Yanga J, et al. Detection methods for Pseudomonas aeruginosa: history and future perspective. RSC Adv. 2017;7:51789–800.
Leung LM, Fondrie WE, Doi Y, Johnson JK, Strickland DK, Ernst RK, et al. Identification of the ESKAPE pathogens by mass spectrometric analysis of microbial membrane glycolipids. Sci Rep. 2017;7(1):6403. https://doi.org/10.1038/s41598-017-04793-4.
Soundy J, Day D. Selection of DNA aptamers specific for live Pseudomonas aeruginosa. PLoS One. 2017;12(9):e0185385. https://doi.org/10.1371/journal.pone.0185385.
Wang KY, Zeng YL, Yang XY, Li WB, Lan XP. Utility of aptamer-fluorescence in situ hybridization for rapid detection of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2011;30(2):273–8. https://doi.org/10.1007/s10096-010-1074-0.
Hu Y, Zou W, Julita V, Ramanathan R, Tabor RF, Nixon-Luke R, et al. Photomodulation of bacterial growth and biofilm formation using carbohydrate-based surfactants. Chem Sci. 2016;7(11):6628–34. https://doi.org/10.1039/c6sc03020c.
Berean KJ, Adetutu EM, Zhen Ou J, Nour M, Nguyen EP, Paull D, et al. A unique in vivo approach for investigating antimicrobial materials utilizing fistulated animals. Sci Rep. 2015;5:11515. https://doi.org/10.1038/srep11515.
Mena KD, Gerba CP. Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol. 2009;201:71–115. https://doi.org/10.1007/978-1-4419-0032-6_3.
Kim HJ, Kim HS, Lee JM, Yoon SS, Yong D. Rapid detection of Pseudomonas aeruginosa and Acinetobacter baumannii harboring bla(VIM-2), bla(IMP-1) and bla(OXA-23) genes by using loop-mediated isothermal amplification methods. Ann Lab Med. 2016;36(1):15–22. https://doi.org/10.3343/alm.2016.36.1.15.
Kim LH, Yu HW, Kim YH, Kim SI, Jang A. Potential of fluorophore labeled aptamers for Pseudomonas aeruginosa detection in drinking water. J Korean Soc Appl Biol Chem. 2013;56:165–71.
Muhamadali H, Subaihi A, Mohammadtaheri M, Xu Y, Ellis DI, Ramanathan R, et al. Rapid, accurate, and comparative differentiation of clinically and industrially relevant microorganisms via multiple vibrational spectroscopic fingerprinting. Analyst. 2016;141(17):5127–36. https://doi.org/10.1039/c6an00883f.
Sharma TK, Bruno JG, Dhiman A (2017) ABCs of DNA aptamer and related assay development. Biotechnol Adv 35(2):275-301 doi:https://doi.org/10.1016/j.biotechadv.2017.01.003.
Sharma TK, Ramanathan R, Weerathunge P, Mohammadtaheri M, Daima HK, Shukla R, et al. Aptamer-mediated ‘turn-off/turn-on’ NanoZyme activity of gold nanoparticles for kanamycin detection. Chem Commun (Camb). 2014;50(100):15856–9. https://doi.org/10.1039/c4cc07275h.
Weerathunge P, Ramanathan R, Shukla R, Sharma TK, Bansal V. Aptamer-controlled reversible inhibition of gold NanoZyme activity for pesticide sensing. Anal Chem. 2014;86(24):11937–41. https://doi.org/10.1021/ac5028726.
Dhiman A, Kalra P, Bansal V, Bruno JG, Sharma TK. Aptamer-based point-of-care diagnostic platforms. Sensors Actuators B Chem. 2017;246:535–53.
Sharma TK, Bruno JG, Cho W. The point behind translation of aptamers for point of care diagnostics aptamer and synthetic antibodies. Aptamer Synth Antibodies. 2016;2:36–42.
Chopra A, Shukla R, Sharma TK. Aptamers as an emerging player in biology. Aptamer Synth Antibodies. 2014;1:1–11.
Chen A, Yang S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens Boelectron. 2015;71:230–42. https://doi.org/10.1016/j.bios.2015.04.041.
Kaur H, Bruno J, Kumar A, Sharma TK. Aptamers in the therapeutics and diagnostics pipelines. Theranostics. 2018;8(15):4016–32.
Kalra P, Dhiman A, Cho WC, Bruno JG, TK S. Simple methods and rational design for enhancing aptamer sensitivity and specificity. Front Mol Biosci. 2018;5(41):1–16.
Shum KT, Lui EL, Wong SC, Yeung P, Sam L, Wang Y, et al. Aptamer-mediated inhibition of Mycobacterium tuberculosis polyphosphate kinase 2. Biochemistry. 2011;50(15):3261–71. https://doi.org/10.1021/bi2001455.
Song KM, Jeong E, Jeon W, Cho M, Ban C. Aptasensor for ampicillin using gold nanoparticle based dual fluorescence-colorimetric methods. Anal Bioanal Chem. 2012;402(6):2153–61. https://doi.org/10.1007/s00216-011-5662-3.
Vivekananda J, Kiel L. Anti-Francisella tularensis DNA aptamers detect tularemia antigen from different subspecies by aptamer-linked immobilized sorbent assay. Lab Investig. 2006;86:610–8.
Zhou J, Rossi JJ. Cell-specific aptamer-mediated targeted drug delivery. Oligonucleotides. 2011;21(1):1–10. https://doi.org/10.1089/oli.2010.0264.
Kumari P, Lavania S, Tyagi S, Dhiman A, Rath D, Anthwal D, et al. A novel aptamer-based test for the rapid and accurate diagnosis of pleural tuberculosis. Anal Biochem. 2018;564-565:80–7. https://doi.org/10.1016/j.ab.2018.10.019.
Lavania S, Das R, Dhiman A, Myneedu VP, Verma A, Singh N, Sharma TK, Tyagi JS. Aptamer-based TB antigen tests for the rapid diagnosis of pulmonary tuberculosis: potential utility in screening for tuberculosis. ACS Infect Dis. 2018;4(12):1718–26.https://doi.org/10.1021/acsinfecdis.8b00201.
Dhiman A, Haldar S, Mishra SK, Sharma N, Bansal A, Ahmad Y, et al. Generation and application of DNA aptamers against HspX for accurate diagnosis of tuberculous meningitis. Tuberculosis. 2018;112:27–36. https://doi.org/10.1016/j.tube.2018.07.004.
Qi C, Cai S, Wang X, Li J, Lian Z, Sun S, et al. Enhanced oxidase/peroxidase-like activities of aptamer conjugated MoS2/PtCu nanocomposites and their biosensing application. RSC Adv. 2016;6:54949–55. https://doi.org/10.1039/C6RA03507H10.1039/x0xx00000x.
Wei H, Wang E. Nanomaterials with enzyme-like characteristics (NanoZymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42(14):6060–93. https://doi.org/10.1039/c3cs35486e.
Zhang L, Li L. Colorimetric thrombin assay using aptamer-functionalized gold nanoparticles acting as a peroxidase mimetic. Microchim Acta. 2015;183(1):485–90. https://doi.org/10.1007/s00604-015-1674-6.
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–83. https://doi.org/10.1038/nnano.2007.260.
Wang X, Hua Y, Wei H. NanoZymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg Chem Front. 2016;3(1):31–60.
Ju H. Functional nanomaterials and nanoprobes for amplified biosensing. Appl Mater Today. 2018;10:51–71.
de la Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol. 2012;7(12):821–4. https://doi.org/10.1038/nnano.2012.186.
Singh M, Weerathunge P, Liyanage PD, Mayes E, Ramanathan R, Bansal V. Competitive inhibition of the enzyme-mimic activity of Gd-based nanorods toward highly specific colorimetric sensing of l-cysteine. Langmuir. 2017;33(38):10006–15. https://doi.org/10.1021/acs.langmuir.7b01926.
Alves-Balvedi RP, Caetano LP, Madurro JM, Brito-Madurro AG. Use of 3,3′,5,5′ tetramethylbenzidine as new electrochemical indicator of DNA hybridization and its application in genossensor. Biosens Bioelectron. 2016;85:226–31. https://doi.org/10.1016/j.bios.2016.05.016.
Luan Y, Chen J, Xie G, Li C, Ping H, Ma Z, et al. Visual and microplate detection of aflatoxin B2 based on NaCl-induced aggregation of aptamer-modified gold nanoparticles. Microchim Acta. 2015;182(5–6):995–10001.
Ng BYC, Wee EJH, West NP, Trau M. Naked-eye colorimetric and electrochemical detection of Mycobacterium tuberculosis—toward rapid screening for active case finding. ACS Sens. 2016;1(2):173–8.
Johansson MA, Hellenäs K. Immunobiosensor analysis of clenbuterol in bovine hair. Food Agric Immunol. 15:197–205.
Linnet K, Kondratovich M. Partly nonparametric approach for determining the limit of detection. Clin Chem. 2004;50(4):732–40. https://doi.org/10.1373/clinchem.2003.029983.
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277(5329):1078–81.
Mirkin C, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–9.
Li H, Rothberg L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc Natl Acad Sci U S A. 2004;101(39):14036–9. https://doi.org/10.1073/pnas.0406115101.
Chen SJ, Huang YF, Huang CC, Lee KH, Lin ZH, Chang HT. Colorimetric determination of urinary adenosine using aptamer-modified gold nanoparticles. Biosens Bioelectron. 2008;23(11):1749–53. https://doi.org/10.1016/j.bios.2008.02.008.
Ng B, Wee EJH, West NP, Trau M. Naked-eye colorimetric and electrochemical detection of Mycobacterium tuberculosis towards rapid screening for active case finding. ACS Sensors. 2015;1(2):173–8.
Carnovalea C, Bryant G, Shukla R, Bansal V. Size, shape and surface chemistry of nano-gold dictate its cellular interactions, uptake and toxicity. Prog Mater Sci. 2016;83:152–90.
Sun S, Zhao R, Feng S, Xie Y. Colorimetric zearalenone assay based on the use of an aptamer and of gold nanoparticles with peroxidase-like activity. Mikrochim Acta. 2018;185(12):535. https://doi.org/10.1007/s00604-018-3078-x.
Yue H, He Y, Fan E, Wang L, Lu S, Fu Z. Label-free electrochemiluminescent biosensor for rapid and sensitive detection of pseudomonas aeruginosa using phage as highly specific recognition agent. Biosens Bioelectron. 2017;94:429–32. https://doi.org/10.1016/j.bios.2017.03.033.
Cattoir V, Gilibert A, Le Glaunec JM, Launay N, Bait-Merabet L, Legrand P. Rapid detection of Pseudomonas aeruginosa from positive blood cultures by quantitative PCR. Ann Clin Microbiol Antimicrob. 2010;9:21. https://doi.org/10.1186/1476-0711-9-21.
Tang Y, Zou J, Ma C, Ali Z, Li Z, Li X, et al. Highly sensitive and rapid detection of Pseudomonas aeruginosa based on magnetic enrichment and magnetic separation. Theranostics. 2013;3(2):85–92. https://doi.org/10.7150/thno.5588.
Krithiga N, Viswanath KB, Vasantha VS, Jayachitra A. Specific and selective electrochemical immunoassay for Pseudomonas aeruginosa based on pectin-gold nano composite. Biosens Bioelectron. 2016;79:121–9. https://doi.org/10.1016/j.bios.2015.12.006.
Thet NT, Jenkins ATA. An electrochemical sensor concept for the detection of bacterial virulence factors from Staphylococcus aureus and Pseudomonas aeruginosa. Electrochem Commun. 2015;59:104–8.
He Y, Wang M, Fan E, Ouyang H, Yue H, Su X, et al. Highly specific bacteriophage-affinity strategy for rapid separation and sensitive detection of viable Pseudomonas aeruginosa. Anal Chem. 2017;89(3):1916–21. https://doi.org/10.1021/acs.analchem.6b04389.
Hu J, Fu K, Bohn PW. Whole-cell Pseudomonas aeruginosa localized surface plasmon resonance aptasensor. Anal Chem. 2018;90(3):2326–32. https://doi.org/10.1021/acs.analchem.7b04800.
Ellairaja S, Krithiga N, Ponmariappan S, Vasantha VS. Novel pyrimidine tagged silver nanoparticle based fluorescent immunoassay for the detection of Pseudomonas aeruginosa. J Agric Food Chem. 2017;65(8):1802–12. https://doi.org/10.1021/acs.jafc.6b04790.
Kaur G, Raj T, Kaur N, Singh N. Pyrimidine-based functional fluorescent organic nanoparticle probe for detection of Pseudomonas aeruginosa. Org Biomol Chem. 2015;13(16):4673–9. https://doi.org/10.1039/c5ob00206k.
Acknowledgements
The authors acknowledge the generous support provided by Prof. Jaya S. Tyagi and Dr. H.K. Prasad in providing laboratory access to handle the bacterial cultures.
Funding
T.K.S. is thankful to THSTI Core grant and the Department of Biotechnology Govt. of India for Innovation Award and Innovative Young Biotechnologist Award (BT/010/IYBA/2016/10). V.B. thanks the Australian Research Council (ARC) for a Future Fellowship (FT140101285) and research support through an ARC Discovery (DP170103477) grant. V.B. also recognizes the generous support of the Ian Potter Foundation toward establishing the Sir Ian Potter NanoBioSensing Facility at RMIT University. A.D. is thankful to the Indian Council for Medical Research for providing Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This research does not involve any human participant or animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 613 kb)
Rights and permissions
About this article
Cite this article
Das, R., Dhiman, A., Kapil, A. et al. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal Bioanal Chem 411, 1229–1238 (2019). https://doi.org/10.1007/s00216-018-1555-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1555-z